Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice
- PMID: 22740171
- PMCID: PMC3402300
- DOI: 10.2337/db11-1711
Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice
Abstract
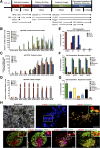
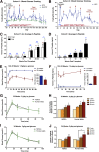
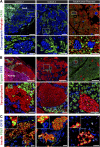
Diabetes is a chronic debilitating disease that results from insufficient production of insulin from pancreatic β-cells. Islet cell replacement can effectively treat diabetes but is currently severely limited by the reliance upon cadaveric donor tissue. We have developed a protocol to efficiently differentiate commercially available human embryonic stem cells (hESCs) in vitro into a highly enriched PDX1+ pancreatic progenitor cell population that further develops in vivo to mature pancreatic endocrine cells. Immature pancreatic precursor cells were transplanted into immunodeficient mice with streptozotocin-induced diabetes, and glycemia was initially controlled with exogenous insulin. As graft-derived insulin levels increased over time, diabetic mice were weaned from exogenous insulin and human C-peptide secretion was eventually regulated by meal and glucose challenges. Similar differentiation of pancreatic precursor cells was observed after transplant in immunodeficient rats. Throughout the in vivo maturation period hESC-derived endocrine cells exhibited gene and protein expression profiles that were remarkably similar to the developing human fetal pancreas. Our findings support the feasibility of using differentiated hESCs as an alternative to cadaveric islets for treating patients with diabetes.
Figures





References
-
- Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature 2001;414:792–798 - PubMed
-
- Ryan EA, Lakey JR, Rajotte RV, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 2001;50:710–719 - PubMed
-
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230–238 - PubMed
-
- Kelly OG, Chan MY, Martinson LA, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol 2011;29:750–756 - PubMed
-
- Jiang J, Au M, Lu K, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 2007;25:1940–1953 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

