The Bin3 RNA methyltransferase targets 7SK RNA to control transcription and translation
- PMID: 22740346
- PMCID: PMC3629726
- DOI: 10.1002/wrna.1123
The Bin3 RNA methyltransferase targets 7SK RNA to control transcription and translation
Abstract
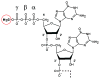
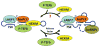
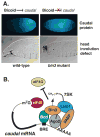
Bicoid-interacting protein 3 (Bin3) is a conserved RNA methyltransferase found in eukaryotes ranging from fission yeast to humans. It was originally discovered as a Bicoid (Bcd)-interacting protein in Drosophila, where it is required for anterior-posterior and dorso-ventral axis determination in the early embryo. The mammalian ortholog of Bin3 (BCDIN3), also known as methyl phosphate capping enzyme (MePCE), plays a key role in repressing transcription. In transcription, MePCE binds the non-coding 7SK RNA, which forms a scaffold for an RNA-protein complex that inhibits positive-acting transcription elongation factor b, an RNA polymerase II elongation factor. MePCE uses S-adenosyl methionine to transfer a methyl group onto the γ-phosphate of the 5' guanosine of 7SK RNA generating an unusual cap structure that protects 7SK RNA from degradation. Bin3/MePCE also has a role in translation regulation. Initial studies in Drosophila indicate that Bin3 targets 7SK RNA and stabilizes a distinct RNA-protein complex that assembles on the 3'-untranslated region of caudal mRNAs to prevent translation initiation. Much remains to be learned about Bin3/MeCPE function, including how it recognizes 7SK RNA, what other RNA substrates it might target, and how widespread a role it plays in gene regulation and embryonic development.
Copyright © 2012 John Wiley & Sons, Ltd.
Conflict of interest statement
The authors declare no Conflict of Interest.
Figures


References
-
- Zhu W. PhD Thesis. Department of Biomedical Sciences, School of Public Health, State University of New York; 2000. Isolation and Characterization of Bicoid-interacting Proteins: Bin1 a Homolog of Human SAP18 and Bin3, a putative Protein Methyltransferase.
-
- Singh N, Morlock H, Hanes SD. The Bin3 RNA methyltransferase is required for repression of caudal translation in the Drosophila embryo. Dev Biol. 2011;352:104–115. - PubMed
-
- Jeronimo C, Forget D, Bouchard A, Li Q, Chua G, Poitras C, Therien C, Bergeron D, Bourassa S, Greenblatt J, Chabot B, Poirier GG, Hughes TR, Blanchette M, Price DH, Coulombe B. Systematic analysis of the protein interaction network for the human transcription machinery reveals the identity of the 7SK capping enzyme. Mol Cell. 2007;27:262–274. - PMC - PubMed
-
- Zhu W, Hanes SD. Identification of Drosophila Bicoid-interacting proteins using a custom two-hybrid selection. Gene. 2000;245:329–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

