Construction and manipulation of a new Kaposi's sarcoma-associated herpesvirus bacterial artificial chromosome clone
- PMID: 22740391
- PMCID: PMC3446615
- DOI: 10.1128/JVI.01019-12
Construction and manipulation of a new Kaposi's sarcoma-associated herpesvirus bacterial artificial chromosome clone
Abstract
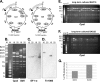
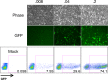
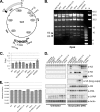
Efficient genetic modification of herpesviruses such as Kaposi's sarcoma-associated herpesvirus (KSHV) has come to rely on bacterial artificial chromosome (BAC) technology. In order to facilitate this approach, we generated a new KSHV BAC clone, called BAC16, derived from the rKSHV.219 virus, which stems from KSHV and Epstein-Barr virus-coinfected JSC1 primary effusion lymphoma (PEL) cells. Restriction enzyme and complete sequencing data demonstrate that the KSHV of JSC1 PEL cells showed a minimal level of sequence variation across the entire viral genome compared to the complete genomic sequence of other KSHV strains. BAC16 not only stably propagated in both Escherichia coli and mammalian cells without apparent genetic rearrangements, but also was capable of robustly producing infectious virions (∼5 × 10(7)/ml). We also demonstrated the utility of BAC16 by generating deletion mutants of either the K3 or K5 genes, whose products are E3 ligases of the membrane-associated RING-CH (MARCH) family. While previous studies have shown that individual expression of either K3 or K5 results in efficient downregulation of the surface expression of major histocompatibility complex class I (MHC-I) molecules, we found that K5, but not K3, was the primary factor critical for the downregulation of MHC-I surface expression during KSHV lytic reactivation or following de novo infection. The data presented here demonstrate the utility of BAC16 for the generation and characterization of KSHV knockout and mutant recombinants and further emphasize the importance of functional analysis of viral genes in the context of the KSHV genome besides the study of individual gene expression.
Figures



References
-
- Barozzi P, et al. 2008. Changes in the immune responses against human herpesvirus-8 in the disease course of posttransplant Kaposi sarcoma. Transplantation 86:738–744 - PubMed
-
- Bartee E, McCormack A, Fruh K. 2006. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2:e107 doi:10.1371/journal.ppat.0020107 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

