Intracytoplasmic trapping of influenza virus by a lipophilic derivative of aglycoristocetin
- PMID: 22740402
- PMCID: PMC3416158
- DOI: 10.1128/JVI.07032-11
Intracytoplasmic trapping of influenza virus by a lipophilic derivative of aglycoristocetin
Abstract
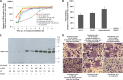
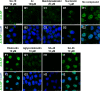
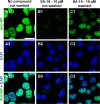
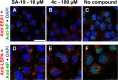
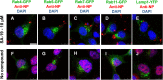
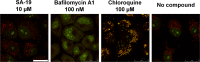
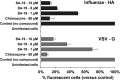
We report on a new anti-influenza virus agent, SA-19, a lipophilic glycopeptide derivative consisting of aglycoristocetin coupled to a phenylbenzyl-substituted cyclobutenedione. In Madin-Darby canine kidney cells infected with influenza A/H1N1, A/H3N2, or B virus, SA-19 displayed a 50% antivirally effective concentration of 0.60 μM and a selectivity index (ratio of cytotoxic versus antiviral concentration) of 112. SA-19 was 11-fold more potent than unsubstituted aglycoristocetin and was active in human and nonhuman cell lines. Virus yield at 72 h p.i. was reduced by 3.6 logs at 0.8 μM SA-19. In contrast to amantadine and oseltamivir, SA-19 did not select for resistance upon prolonged virus exposure. SA-19 was shown to inhibit an early postbinding step in virus replication. The compound had no effect on hemagglutinin (HA)-mediated membrane fusion in an HA-polykaryon assay and did not inhibit the low-pH-induced refolding of the HA in a tryptic digestion assay. However, a marked inhibitory effect on the transduction exerted by retroviral pseudoparticles carrying an HA or vesicular stomatitis virus glycoprotein (VSV-G) fusion protein was noted, suggesting that SA-19 targets a cellular factor with a role in influenza virus and VSV entry. Using confocal microscopy with antinucleoprotein staining, SA-19 was proven to completely prevent the influenza virus nuclear entry. This virus arrest was characterized by the formation of cytoplasmic aggregates. SA-19 appeared to disturb the endocytic uptake and trap the influenza virus in vesicles distinct from early, late, or recycling endosomes. The aglycoristocetin derivative SA-19 represents a new class of potent and broad-acting influenza virus inhibitors with potential clinical relevance.
Figures


References
-
- Balzarini J, et al. 2006. Inhibition of feline (FIPV) and human (SARS) coronavirus by semisynthetic derivatives of glycopeptide antibiotics. Antiviral Res. 72:20–33 doi:10.1016/j.antiviral.2006.03.005 - DOI - PMC - PubMed
-
- Balzarini J, et al. 2003. Antiretroviral activity of semisynthetic derivatives of glycopeptide antibiotics. J. Med. Chem. 46:2755–2764 - PubMed
-
- Bardsley B, Williams DH, Baglin TP. 1998. Cleavage of rhamnose from ristocetin A removes its ability to induce platelet aggregation. Blood Coagul. Fibrinolysis 9:241–244 - PubMed
-
- Bauer K, Richter M, Wutzler P, Schmidtke M. 2009. Different neuraminidase inhibitor susceptibilities of human H1N1, H1N2, and H3N2 influenza A viruses isolated in Germany from 2001 to 2005/2006. Antiviral Res. 82:34–41 - PubMed
-
- Belser JA, et al. 2007. DAS181, a novel sialidase fusion protein, protects mice from lethal avian influenza H5N1 virus infection. J. Infect. Dis. 196:1493–1499 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

