Quantitative measurement of human papillomavirus type 16 e5 oncoprotein levels in epithelial cell lines by mass spectrometry
- PMID: 22740411
- PMCID: PMC3416135
- DOI: 10.1128/JVI.01032-12
Quantitative measurement of human papillomavirus type 16 e5 oncoprotein levels in epithelial cell lines by mass spectrometry
Abstract
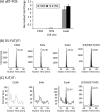
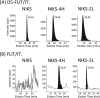
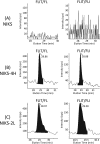
The high-risk human papillomavirus type 16 (HPV-16) E5 protein (16E5) induces tumors in a transgenic mouse model and may contribute to early stages of cervical carcinogenesis. Although high-risk E5 expression is generally thought to be lost during the progression to cervical carcinoma following integration of HPV DNA into the host genome, episomal viral DNA has been documented in a subset of HPV-16-positive malignant lesions. Numerous studies have shown that transcripts that could potentially encode 16E5 are present in cervical biopsy specimens and cervical cancer cell lines, but the presence of E5 protein has been demonstrated in only two reports that have not been corroborated. In the present study, we show that trypsin cleavage of 16E5 generates a unique four-amino-acid C-terminal peptide (FLIT) that serves as a marker for E5 expression in transfected cells and epithelial cell lines containing integrated and episomal HPV-16 DNA. Following trypsin cleavage, reversed-phase chromatography and mass spectrometry (MS) were used to detect FLIT. Immunoprecipitation assays using a newly generated anti-16E5 antibody confirmed that 16E5 was solely responsible for the FLIT signal, and deuterated FLIT peptide provided an internal standard that enabled us to quantify the number of 16E5 molecules per cell. We show that 16E5 is expressed in the Caski but not in the SiHa cervical cancer cell line, suggesting that 16E5 may contribute to the malignant phenotype of some cervical cancers, even in cells exclusively containing an integrated HPV genome.
Figures





Similar articles
-
Expression of the E5 Oncoprotein of HPV16 Impacts on the Molecular Profiles of EMT-Related and Differentiation Genes in Ectocervical Low-Grade Lesions.Int J Mol Sci. 2021 Jun 18;22(12):6534. doi: 10.3390/ijms22126534. Int J Mol Sci. 2021. PMID: 34207106 Free PMC article.
-
The Expression of HPV-16 E5 Oncoprotein Impacts the Transcript Profiles of FGFR2 and EMT-Related Genes in Preneoplastic Anal Epithelium Lesions.Int J Mol Sci. 2024 Nov 11;25(22):12085. doi: 10.3390/ijms252212085. Int J Mol Sci. 2024. PMID: 39596152 Free PMC article.
-
[Effects of human papillomavirus 16 E5 on malignancy of human cervical cancer cells and the mechanism there of].Zhonghua Yi Xue Za Zhi. 2008 Nov 25;88(43):3080-4. Zhonghua Yi Xue Za Zhi. 2008. PMID: 19192411 Chinese.
-
Cellular Functions of HPV16 E5 Oncoprotein during Oncogenic Transformation.Mol Cancer Res. 2021 Feb;19(2):167-179. doi: 10.1158/1541-7786.MCR-20-0491. Epub 2020 Oct 26. Mol Cancer Res. 2021. PMID: 33106372 Review.
-
Advances in molecular mechanism of HPV16 E5 oncoprotein carcinogenesis.Arch Biochem Biophys. 2023 Sep 1;745:109716. doi: 10.1016/j.abb.2023.109716. Epub 2023 Aug 6. Arch Biochem Biophys. 2023. PMID: 37553047 Review.
Cited by
-
Development of Novel Single-Chain Antibodies against the Hydrophobic HPV-16 E5 Protein.Biomed Res Int. 2018 Jun 20;2018:5809028. doi: 10.1155/2018/5809028. eCollection 2018. Biomed Res Int. 2018. PMID: 30027096 Free PMC article.
-
HPV Detection in Breast Tumors and Associated Risk Factors in Northeastern Brazil.Cells. 2024 Jun 29;13(13):1132. doi: 10.3390/cells13131132. Cells. 2024. PMID: 38994984 Free PMC article.
-
Human papillomavirus type 16 E5-mediated upregulation of Met in human keratinocytes.Virology. 2018 Jun;519:1-11. doi: 10.1016/j.virol.2018.03.021. Epub 2018 Mar 31. Virology. 2018. PMID: 29609071 Free PMC article.
-
HPV 16 E5 oncoprotein is expressed in early stage carcinogenesis and can be a target of immunotherapy.Hum Vaccin Immunother. 2017 Feb;13(2):291-297. doi: 10.1080/21645515.2017.1264777. Epub 2016 Dec 8. Hum Vaccin Immunother. 2017. PMID: 27929754 Free PMC article.
-
Alkyl-imino sugars inhibit the pro-oncogenic ion channel function of human papillomavirus (HPV) E5.Antiviral Res. 2018 Oct;158:113-121. doi: 10.1016/j.antiviral.2018.08.005. Epub 2018 Aug 7. Antiviral Res. 2018. PMID: 30096339 Free PMC article.
References
-
- Allen-Hoffmann BL, et al. 2000. Norman growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J. Invest. Dermatol. 114:444–455 - PubMed
-
- Ashrafi GH, Haghshenas M, Marchetti B, Campo MS. 2006. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int. J. Cancer 119:2105–2112 - PubMed
-
- Baege AC, Berger A, Schlegel R, Veldman T, Schlegel R. 2002. Cervical epithelial cells transduced with the papillomavirus E6/E7 oncogenes maintain stable levels of oncoprotein expression but exhibit progressive, major increases in hTERT gene expression and telomerase activity. Am. J. Pathol. 160:1251–1257 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

