NAC functions as a modulator of SRP during the early steps of protein targeting to the endoplasmic reticulum
- PMID: 22740632
- PMCID: PMC3418300
- DOI: 10.1091/mbc.E12-02-0112
NAC functions as a modulator of SRP during the early steps of protein targeting to the endoplasmic reticulum
Abstract
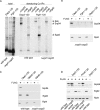
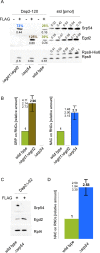
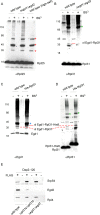
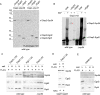
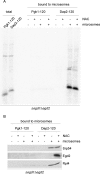
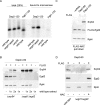
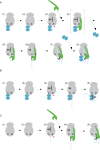
Nascent polypeptide-associated complex (NAC) was initially found to bind to any segment of the nascent chain except signal sequences. In this way, NAC is believed to prevent mistargeting due to binding of signal recognition particle (SRP) to signalless ribosome nascent chain complexes (RNCs). Here we revisit the interplay between NAC and SRP. NAC does not affect SRP function with respect to signalless RNCs; however, NAC does affect SRP function with respect to RNCs targeted to the endoplasmic reticulum (ER). First, early recruitment of SRP to RNCs containing a signal sequence within the ribosomal tunnel is NAC dependent. Second, NAC is able to directly and tightly bind to nascent signal sequences. Third, SRP initially displaces NAC from RNCs; however, when the signal sequence emerges further, trimeric NAC·RNC·SRP complexes form. Fourth, upon docking to the ER membrane NAC remains bound to RNCs, allowing NAC to shield cytosolically exposed nascent chain domains not only before but also during cotranslational translocation. The combined data indicate a functional interplay between NAC and SRP on ER-targeted RNCs, which is based on the ability of the two complexes to bind simultaneously to distinct segments of a single nascent chain.
Figures

Similar articles
-
Binding of signal recognition particle gives ribosome/nascent chain complexes a competitive advantage in endoplasmic reticulum membrane interaction.Mol Biol Cell. 1998 Jan;9(1):103-15. doi: 10.1091/mbc.9.1.103. Mol Biol Cell. 1998. PMID: 9436994 Free PMC article.
-
The intrinsic ability of ribosomes to bind to endoplasmic reticulum membranes is regulated by signal recognition particle and nascent-polypeptide-associated complex.Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9435-9. doi: 10.1073/pnas.92.21.9435. Proc Natl Acad Sci U S A. 1995. PMID: 7568149 Free PMC article.
-
A signal-anchor sequence stimulates signal recognition particle binding to ribosomes from inside the exit tunnel.Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1398-403. doi: 10.1073/pnas.0808584106. Epub 2009 Jan 21. Proc Natl Acad Sci U S A. 2009. PMID: 19164516 Free PMC article.
-
Fidelity of Cotranslational Protein Targeting to the Endoplasmic Reticulum.Int J Mol Sci. 2021 Dec 28;23(1):281. doi: 10.3390/ijms23010281. Int J Mol Sci. 2021. PMID: 35008707 Free PMC article. Review.
-
Co-translational targeting and translocation of proteins to the endoplasmic reticulum.Biochim Biophys Acta. 2013 Nov;1833(11):2392-402. doi: 10.1016/j.bbamcr.2013.02.021. Epub 2013 Feb 26. Biochim Biophys Acta. 2013. PMID: 23481039 Review.
Cited by
-
The biological functions of Naa10 - From amino-terminal acetylation to human disease.Gene. 2015 Aug 10;567(2):103-31. doi: 10.1016/j.gene.2015.04.085. Epub 2015 May 16. Gene. 2015. PMID: 25987439 Free PMC article. Review.
-
The role of the E3 ligase Not4 in cotranslational quality control.Front Genet. 2014 May 19;5:141. doi: 10.3389/fgene.2014.00141. eCollection 2014. Front Genet. 2014. PMID: 24904641 Free PMC article. Review.
-
Mechanism of signal sequence handover from NAC to SRP on ribosomes during ER-protein targeting.Science. 2022 Feb 25;375(6583):839-844. doi: 10.1126/science.abl6459. Epub 2022 Feb 24. Science. 2022. PMID: 35201867 Free PMC article.
-
Sse1, Hsp110 chaperone of yeast, controls the cellular fate during endoplasmic reticulum stress.G3 (Bethesda). 2024 Jun 5;14(6):jkae075. doi: 10.1093/g3journal/jkae075. G3 (Bethesda). 2024. PMID: 38577891 Free PMC article.
-
Visualization of cytosolic ribosomes on the surface of mitochondria by electron cryo-tomography.EMBO Rep. 2017 Oct;18(10):1786-1800. doi: 10.15252/embr.201744261. Epub 2017 Aug 21. EMBO Rep. 2017. PMID: 28827470 Free PMC article.
References
-
- Bukau B, Deuerling E, Pfund C, Craig EA. Getting newly synthesized proteins into shape. Cell. 2000;101:119–122. - PubMed
-
- Cheng Z, Gilmore R. Slow translocon gating causes cytosolic exposure of transmembrane and lumenal domains during membrane protein integration. Nat Struct Mol Biol. 2006;13:930–936. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

