A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4⁺ T-cell recovery despite sustained virologic suppression: ACTG A5256
- PMID: 22740718
- PMCID: PMC3491731
- DOI: 10.1093/infdis/jis376
A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4⁺ T-cell recovery despite sustained virologic suppression: ACTG A5256
Abstract
Background: Despite viral suppression, antiretroviral therapy (ART) does not restore CD4(+) T-cell counts in many patients infected with human immunodeficiency virus type 1 (HIV-1).
Methods: In a single-arm pilot trial involving ART recipients with suppressed plasma levels of HIV-1 RNA for at least 48 weeks and stable suboptimal CD4(+) T-cell recovery, subjects added maraviroc, a CCR5 antagonist, to their existing ART for 24 weeks. After stopping maraviroc, they were followed for an additional 24 weeks. A Wilcoxon signed-rank test was used to evaluate whether maraviroc was associated with an increase of at least 20 cells/µL in the CD4(+) T-cell count.
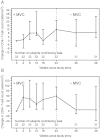
Results: A total of 34 subjects were enrolled. The median age was 50 years, and the median baseline CD4(+) T-cell count was 153 cells/µL. The median increase in CD4(+) T-cell count from baseline to week 22/24 was 12 cells/µL (90% confidence interval, 1-22). A CD4(+) T-cell count increase of at least 20 cells/µL was not detected (P = .97). Markers of immune activation and apoptosis decreased during maraviroc intensification; this decline partially reversed after discontinuing maraviroc.
Conclusions: Adding maraviroc to suppressive ART for 24 weeks was not associated with an increase in CD4(+) T-cell counts of at least 20 cells/µL. Further studies of CCR5 antagonists in the dampening of immune activation associated with HIV infection are warranted. Clinical Trials Registration. NCT 00709111.
Trial registration: ClinicalTrials.gov NCT00709111.
Figures


References
-
- Bartlett JA, Fath MJ, Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20:2051–64. - PubMed
-
- Grabar S, Le Moing V, Goujard C, et al. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–10. - PubMed
-
- Chene G, Sterne JA, May M, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–86. - PubMed
-
- Lodwick RK, Sabin CA, et al. Study Group on Death Rates at High CD4 Count in Antiretroviral Naive Patients. Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per microL in Europe and North America: a pooled cohort observational study. Lancet. 2010;376:340–5. - PMC - PubMed
-
- Bruyand M, Thiebaut R, Lawson-Ayayi S, et al. Paper presented at: 15th Conference on Retroviruses and Opportunistic Infections; 4 February 2008; Boston, MA. Immunodeficiency and risk of AIDS-defining and non–AIDS-defining cancers: ANRS CO3 Aquitaine Cohort, 1998 to 2006 [abstract 15]
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- G08 LM008830/LM/NLM NIH HHS/United States
- AI069452/AI/NIAID NIH HHS/United States
- AI069484/AI/NIAID NIH HHS/United States
- UM1 AI069556/AI/NIAID NIH HHS/United States
- U01 AI069477/AI/NIAID NIH HHS/United States
- U01 AI069502/AI/NIAID NIH HHS/United States
- RR023561/RR/NCRR NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- AI050410/AI/NIAID NIH HHS/United States
- UM1 AI069503/AI/NIAID NIH HHS/United States
- K24 AI51966/AI/NIAID NIH HHS/United States
- U01 AI069474/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- AI069465/AI/NIAID NIH HHS/United States
- AI069556/AI/NIAID NIH HHS/United States
- U01 AI069447/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- UM1 AI069472/AI/NIAID NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- AI069419/AI/NIAID NIH HHS/United States
- AI069511/AI/NIAID NIH HHS/United States
- RR024160/RR/NCRR NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- AI69501/AI/NIAID NIH HHS/United States
- K08 AI081545/AI/NIAID NIH HHS/United States
- U01 AI069465/AI/NIAID NIH HHS/United States
- P30 AI082151/AI/NIAID NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- AI082151/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- AI069503/AI/NIAID NIH HHS/United States
- AI069532/AI/NIAID NIH HHS/United States
- AI069474/AI/NIAID NIH HHS/United States
- R01 AI066992/AI/NIAID NIH HHS/United States
- UM1 AI069495/AI/NIAID NIH HHS/United States
- U01 AI069484/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069556/AI/NIAID NIH HHS/United States
- U01 AI069418/AI/NIAID NIH HHS/United States
- AI069502/AI/NIAID NIH HHS/United States
- TL1 RR024978/RR/NCRR NIH HHS/United States
- AI069423/AI/NIAID NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- K24 AI051966/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- KL2 RR024977/RR/NCRR NIH HHS/United States
- G08LM008830-01/LM/NLM NIH HHS/United States
- AI069439/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI069484/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- AI069495/AI/NIAID NIH HHS/United States
- U01 AI68636/AI/NIAID NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- RR024975/RR/NCRR NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069474/AI/NIAID NIH HHS/United States
- AI060354/AI/NIAID NIH HHS/United States
- RR 025747/RR/NCRR NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- AI069424/AI/NIAID NIH HHS/United States
- UL1 RR024160/RR/NCRR NIH HHS/United States
- U01 AI069495/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- U01 AI069503/AI/NIAID NIH HHS/United States
- AI069418/AI/NIAID NIH HHS/United States
- UM1 AI069502/AI/NIAID NIH HHS/United States
- AI069494/AI/NIAID NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- U01 AI069424/AI/NIAID NIH HHS/United States
- AI050409/AI/NIAID NIH HHS/United States
- AI045008/AI/NIAID NIH HHS/United States
- R01 AI066992‐04A1/AI/NIAID NIH HHS/United States
- AI069472/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- AI069432/AI/NIAID NIH HHS/United States
- AI068634/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- AI069447/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069447/AI/NIAID NIH HHS/United States
- U01 AI069452/AI/NIAID NIH HHS/United States
- AI069477/AI/NIAID NIH HHS/United States
- RR024996/RR/NCRR NIH HHS/United States
- AI069471/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

