Chemically modified heparins inhibit fibrinogen-bridged indirect adhesion between tumor cells and platelets
- PMID: 22740939
- PMCID: PMC3362413
- DOI: 10.3892/ol.2011.510
Chemically modified heparins inhibit fibrinogen-bridged indirect adhesion between tumor cells and platelets
Abstract
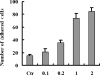
The interaction between platelets and tumor cells is critical for the hematogenous metastasis of tumor cells. We recently reported that fibrinogen was capable of bridging and enhancing the interaction of platelets and tumor cells under conditions of physical shear force. In the present study, we aimed to detect the effects of 8 chemically modified heparins on the binding of fibrinogen to platelets or tumor cells using flow cytometry assays, as well as the fibrinogen-bridged adhesion of platelets and tumor cells using flow chamber assays. The results showed that fibrinogen binds to platelets and tumor cells in a β3 integrin-dependent manner and bridges the adhesion between platelets and tumor cells. Heparin and certain chemically modified heparins, including borohydride-reduced (RO)-, carboxyl-reduced (CR)- and 2-O, 3-O-desulfated (2/3ODS)-heparins, inhibited the β3 integrin-dependent adhesion of fibrinogen to platelets or tumor cells, and consequently blocked the fibrinogen-bridged indirect adhesion of platelets to tumor cells. These data indicate that chemically modified heparins should be potential inhibitors for the fibrinogen-bridged indirect adhesion of platelets and tumor cells, which provides a novel explanation of the anti-adhesion property of heparin and proposes a new anti-metastatic target for cancer treatment.
Figures

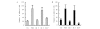
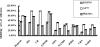
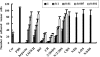
References
-
- Weiler H. A platelet cloak for tumor cells. Blood. 2005;105:5–6.
-
- Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–1300. - PubMed
-
- Manegold PC, Hutter J, Pahernik SA, Messmer K, Dellian M. Platelet-endothelial interaction in tumor angiogenesis and microcirculation. Blood. 2003;101:1970–1976. - PubMed
-
- Trikha M, Nakada MT. Platelets and cancer: implications for antiangiogenic therapy. Semin Thromb Hemost. 2002;28:39–44. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
