Light-induced Ca(2+) transients observed in widefield epi-fluorescence microscopy of excitable cells
- PMID: 22741073
- PMCID: PMC3370967
- DOI: 10.1364/BOE.3.001266
Light-induced Ca(2+) transients observed in widefield epi-fluorescence microscopy of excitable cells
Abstract
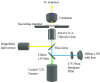
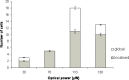
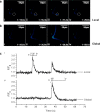
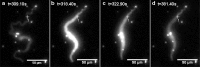
We have investigated the possibility that variations in the level of intracellular Ca(2+) in excitable cells might be induced as an artifact of the incoherent illumination that is being used to monitor transient responses. In order to avoid the fluctuations in power of an arc lamp source, a microscope using a light emitting diode that was calibrated accurately at low power levels, was constructed to provide good control over the dose of light applied to the biological specimen. We report here that higher powers of illumination increased the probability of occurrence of Ca(2+) transients even in the sub-mW range normally used to measure such transients in epi-fluorescence work, suggesting that caution should be exercised when designing experiments and interpreting data.
Keywords: (170.0110) Imaging systems; (170.2520) Fluorescence microscopy; (170.3880) Medical and biological imaging; (170.5380) Physiology.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous
