Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols
- PMID: 22741609
- PMCID: PMC3408084
- DOI: 10.1021/ja3009693
Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols
Abstract
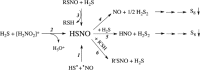
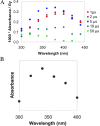
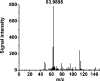
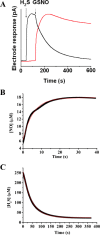
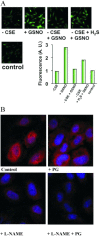
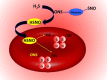
Dihydrogen sulfide recently emerged as a biological signaling molecule with important physiological roles and significant pharmacological potential. Chemically plausible explanations for its mechanisms of action have remained elusive, however. Here, we report that H(2)S reacts with S-nitrosothiols to form thionitrous acid (HSNO), the smallest S-nitrosothiol. These results demonstrate that, at the cellular level, HSNO can be metabolized to afford NO(+), NO, and NO(-) species, all of which have distinct physiological consequences of their own. We further show that HSNO can freely diffuse through membranes, facilitating transnitrosation of proteins such as hemoglobin. The data presented in this study explain some of the physiological effects ascribed to H(2)S, but, more broadly, introduce a new signaling molecule, HSNO, and suggest that it may play a key role in cellular redox regulation.
Figures



References
-
- Koppenol W. H. Free Radical Biol. Med. 1998, 25, 385–391. - PubMed
- Ford P. C.; Lorkovic I. Chem. Rev. 2002, 102, 993–1018. - PubMed
- McCleverty J. A. Chem. Rev. 2002, 102, 403–418. - PubMed
- Tennyson A. G.; Lippard S. J. Chem. Biol. 2011, 18, 1211–1220. - PubMed
- Stamler J. S.; Singel D. J.; Loscalzo J. Science 1992, 258, 1898–1902. - PubMed
- Moncada S.; Palmer R. M. J.; Higgs E. A. Pharmacol. Rev. 1991, 43, 109–142. - PubMed
- Calabrese V.; Cornelius C.; Rizzarelli E.; Owen J. B.; Dinkova-Kostova A. T.; Butterfield D. A. Antioxid. Redox Signaling 2009, 11, 2717–2739. - PubMed
- Palmer R. M. J.; Ferrige A. G.; Moncada S. Nature 1987, 327, 524–526. - PubMed
-
- Whiteman M.; Li L.; Kostetski I.; Chu S. H.; Siau J. L.; Bhatia M.; Moore P. K. Biochem. Biophys. Res. Commun. 2006, 343, 303–310. - PubMed
- Yong Q. C.; Hu L. F.; Wang S.; Huang D.; Bian J. S. Cardiovasc. Res. 2010, 88, 482–491. - PubMed
- Ondrias K.; Stasko A.; Cacanyiova S.; Sulova Z.; Krizanova O.; Kristek F.; Malekova L.; Knezl V.; Breier A. Pfluegers Arch. 2008, 457, 271–279. - PubMed
- Sojitra B.; Bulani Y.; Putcha U. K.; Kanwal A.; Gupta P.; Kuncha M.; Banerjee S. K. Mol. Cell. Biochem. 2012, 60, 61–69. - PubMed
- Bertova A.; Cacanyiova S.; Kristek F.; Krizanova O.; Ondrias K.; Tomaskova Z. Gen. Physiol. Biophys. 2010, 29, 402–410. - PubMed
-
- Zhao W.; Wang R. Am. J. Physiol.: Heart Circ. Physiol. 2002, 283, H474–H480. - PubMed
- Mustafa A. K.; Sikka G.; Gazi S. K.; Steppan J.; Jung S. M.; Bhunia A. K.; Barodka V. M.; Gazi F. K.; Barrow R. K.; Wang R.; Amzel L. M.; Berkowitz D. E.; Snyder S. H. Circ. Res. 2011, 109, 1259–1268. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

