The efficacy and tolerability of artemisinin-piperaquine (Artequick®) versus artesunate-amodiaquine (Coarsucam™) for the treatment of uncomplicated Plasmodium falciparum malaria in south-central Vietnam
- PMID: 22741618
- PMCID: PMC3411481
- DOI: 10.1186/1475-2875-11-217
The efficacy and tolerability of artemisinin-piperaquine (Artequick®) versus artesunate-amodiaquine (Coarsucam™) for the treatment of uncomplicated Plasmodium falciparum malaria in south-central Vietnam
Abstract
Background: In Vietnam, the artemisinin-based combination therapy (ACT) of dihydroartemisinin-piperaquine is currently used for first-line treatment of uncomplicated Plasmodium falciparum malaria. However, limited efficacy and tolerability data are available on alternative forms of ACT in Vietnam in case there is a reduction in the susceptibility of dihydroartemisinin-piperaquine. A study was conducted to compare the efficacy and tolerability of two fixed-dose formulations of ACT, artemisinin-piperaquine (Artequick®, ARPQ) and artesunate-amodiaquine (Coarsucam™, ASAQ) for the treatment of P. falciparum malaria in south-central Vietnam.
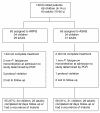
Methods: A randomized, open-label trial was conducted comparing the efficacy of a two-day regimen of ARPQ (~2.8 mg/kg artemisinin plus ~17.1 mg/kg of piperaquine per day) and a three-day regimen of ASAQ (~4.7 mg/kg of artesunate plus ~12.6 mg/kg of amodiaquine per day) for the treatment of children and adults with uncomplicated falciparum malaria. Primary efficacy endpoint was day 42, PCR-corrected, parasitological cure rate. Secondary endpoints were parasite and fever clearance times and tolerability.
Results: Of 128 patients enrolled, 63 were administered ARPQ and 65 ASAQ. Of the patients who completed the 42 days follow-up period or had a recurrence of malaria, 55 were on ARPQ (30 children, 25 adults) and 59 were on ASAQ (31 children, 28 adults). Recrudescent parasitaemia was PCR-confirmed for one patient in each treatment group, with cure rates at day 42 of 98% (95% CI: 88-100) for both forms of ACT. The median parasite clearance time was significantly slower in the ARPQ group compared with the ASAQ group (48 h vs. 36 h, P<0.001) and fever clearance times were shorter in the ASAQ group (12 h vs. 24 h, P=0.07). The two forms of ACT were well tolerated with no serious adverse events.
Conclusion: Both forms of ACT were highly efficacious in the treatment of uncomplicated P. falciparum malaria. Although the two-day course of ARPQ was equally as effective as the three-day course of ASAQ, parasite and fever clearance times were shorter with ASAQ. Further studies are warranted in different regions of Vietnam to determine the nationwide efficacy of ASAQ.
Trial registration: Australian New Zealand Clinical Trials Registry Number, ACTRN12609000816257.
Figures
Similar articles
-
Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial.Lancet. 2020 Apr 25;395(10233):1345-1360. doi: 10.1016/S0140-6736(20)30552-3. Epub 2020 Mar 11. Lancet. 2020. PMID: 32171078 Free PMC article. Clinical Trial.
-
Randomized, multicentre assessment of the efficacy and safety of ASAQ--a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria.Malar J. 2009 Jun 8;8:125. doi: 10.1186/1475-2875-8-125. Malar J. 2009. PMID: 19505304 Free PMC article. Clinical Trial.
-
Repeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trial.Malar J. 2011 Aug 12;10:237. doi: 10.1186/1475-2875-10-237. Malar J. 2011. PMID: 21838909 Free PMC article. Clinical Trial.
-
Therapeutic efficacy of artemether-lumefantrine, artesunate-amodiaquine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Sub-Saharan Africa: A systematic review and meta-analysis.PLoS One. 2022 Mar 10;17(3):e0264339. doi: 10.1371/journal.pone.0264339. eCollection 2022. PLoS One. 2022. PMID: 35271592 Free PMC article.
-
Dihydroartemisinin/Piperaquine: a review of its use in the treatment of uncomplicated Plasmodium falciparum malaria.Drugs. 2012 May 7;72(7):937-61. doi: 10.2165/11203910-000000000-00000. Drugs. 2012. PMID: 22515619 Review.
Cited by
-
Population pharmacokinetic properties of artemisinin in healthy male Vietnamese volunteers.Malar J. 2016 Feb 16;15:90. doi: 10.1186/s12936-016-1134-8. Malar J. 2016. PMID: 26879816 Free PMC article. Clinical Trial.
-
Multinormal in vitro distribution of Plasmodium falciparum susceptibility to piperaquine and pyronaridine.Malar J. 2015 Feb 5;14:49. doi: 10.1186/s12936-015-0586-6. Malar J. 2015. PMID: 25848972 Free PMC article.
-
The effect of dosing strategies on the therapeutic efficacy of artesunate-amodiaquine for uncomplicated malaria: a meta-analysis of individual patient data.BMC Med. 2015 Mar 31;13:66. doi: 10.1186/s12916-015-0301-z. BMC Med. 2015. PMID: 25888957 Free PMC article.
-
In Vivo Efficacy and Tolerability of Artesunate-Azithromycin for the Treatment of Falciparum Malaria in Vietnam.Am J Trop Med Hyg. 2016 Jul 6;95(1):164-7. doi: 10.4269/ajtmh.16-0144. Epub 2016 May 23. Am J Trop Med Hyg. 2016. PMID: 27215294 Free PMC article. Clinical Trial.
-
Analysis of anti-malarial resistance markers in pfmdr1 and pfcrt across Southeast Asia in the Tracking Resistance to Artemisinin Collaboration.Malar J. 2016 Nov 8;15(1):541. doi: 10.1186/s12936-016-1598-6. Malar J. 2016. PMID: 27825353 Free PMC article. Clinical Trial.
References
-
- World Health Organization. World malaria report. , ; 2011. http://www.who.int/mediacentre/factsheets/fs094/en/index.html.
-
- World Health Organization. Guidelines for the treatment of malaria. 2. WHO, Geneva; 2010. - PubMed
-
- Myint HY, Ashley EA, Day NP, Nosten F, White NJ. Efficacy and safety of dihydroartemisinin-piperaquine. Trans R Soc Trop Med Hyg. 2007;101:858–866. - PubMed
-
- Valecha N, Phyo AP, Mayxay M, Newton PN, Krudsood S, Keomany S, Khanthavong M, Pongvongsa T, Ruangveerayuth R, Uthaisil C, Ubben D, Duparc S, Bacchieri A, Corsi M, Rao BH, Bhattacharya PC, Dubhashi N, Ghosh SK, Dev V, Kumar A, Pukittayakamee S. An open-label, randomised study of dihydroartemisinin-piperaquine versus artesunate-mefloquine for falciparum malaria in Asia. PLoS One. 2010;5:e11880. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical