Evolutionary divergence of sedoheptulose 7-phosphate cyclases leads to several distinct cyclic products
- PMID: 22741921
- PMCID: PMC3405187
- DOI: 10.1021/ja3041866
Evolutionary divergence of sedoheptulose 7-phosphate cyclases leads to several distinct cyclic products
Abstract
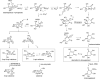
Sedoheptulose 7-phosphate cyclases are enzymes that utilize the pentose phosphate pathway intermediate, sedoheptulose 7-phosphate, to generate cyclic precursors of many bioactive natural products, such as the antidiabetic drug acarbose, the crop protectant validamycin, and the natural sunscreens mycosporine-like amino acids. These proteins are phylogenetically related to the dehydroquinate (DHQ) synthases from the shikimate pathway and are part of the more recently recognized superfamily of sugar phosphate cyclases, which includes DHQ synthases, aminoDHQ synthases, and 2-deoxy-scyllo-inosose synthases. Through genome mining and biochemical studies, we identified yet another subset of DHQS-like proteins in the actinomycete Actinosynnema mirum and the myxobacterium Stigmatella aurantiaca DW4/3-1. These enzymes catalyze the conversion of sedoheptulose 7-phosphate to 2-epi-valiolone, which is predicted to be an alternative precursor for aminocyclitol biosynthesis. Comparative bioinformatics and biochemical analyses of these proteins with 2-epi-5-epi-valiolone synthases (EEVS) and desmethyl-4-deoxygadusol synthases (DDGS) provided further insights into their genetic diversity, conserved amino acid sequences, and plausible catalytic mechanisms. The results further highlight the uniquely diverse DHQS-like sugar phosphate cyclases, which may provide new tools for chemoenzymatic, stereospecific synthesis of various cyclic molecules.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

