Methadone induction in primary care (ANRS-Methaville): a phase III randomized intervention trial
- PMID: 22741944
- PMCID: PMC3528472
- DOI: 10.1186/1471-2458-12-488
Methadone induction in primary care (ANRS-Methaville): a phase III randomized intervention trial
Abstract
Background: In France, the rapid scale-up of buprenorphine, an opioid maintenance treatment (OMT), in primary care for drug users has led to an impressive reduction in HIV prevalence among injecting drug users (IDU) but has had no major effect on Hepatitis C incidence. To date, patients willing to start methadone can only do so in a methadone clinic (a medical centre for drug and alcohol dependence (CSAPA) or a hospital setting) and are referred to primary care physicians after dose stabilization. This study aims to assess the effectiveness of methadone in patients who initiated treatment in primary care compared with those who initiated it in a CSAPA, by measuring abstinence from street opioid use after one year of treatment.
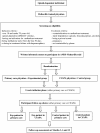
Methods/design: The ANRS-Methaville study is a randomized multicenter non-inferiority control trial comparing methadone induction (lasting approximately 2 weeks) in primary care and in CSAPA. The model of care chosen for methadone induction in primary care was based on study-specific pre-training of all physicians, exclusion criteria and daily supervision of methadone during the initiation phase. Between January 2009 and January 2011, 10 sites each having one CSAPA and several primary care physicians, were identified to recruit patients to be randomized into two groups, one starting methadone in primary care (n = 147), the other in CSAPA (n = 48). The primary outcome of the study is the proportion of participants abstinent from street opioids after 1 year of treatment i.e. non-inferiority of primary care model in terms of the proportion of patients not using street opioids compared with the proportion observed in those starting methadone in a CSAPA.
Discussion: The ANRS-Methaville study is the first in France to use an interventional trial to improve access to OMT for drug users. Once the non-inferiority results become available, the Ministry of Health and agency for the safety of health products may change the the New Drug Application (NDA) of methadone and make methadone induction by trained primary care physicians possible.The trial is registered with the French Agency of Pharmaceutical Products (AFSSAPS) under the number 2008-A0277-48, the European Union Drug Regulating Authorities Clinical Trials.Number Eudract 2008-001338-28, the ClinicalTrials.gov Identifier: NCT00657397 and the International Standard Randomised Controlled Trial Number Register ISRCTN31125511.
Figures
References
-
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008. p. CD002207. - PubMed
-
- Michel L, Roux P, Cohen J, Carrieri MP. A French prospective observational study of the treatment of chronic hepatitis C in drug abusers: a commentary. Gastroenterol Clin Biol. 2009;33(1 Pt 1):15–16. author reply 16–17. - PubMed
-
- Carrieri MP, Amass L, Lucas GM, Vlahov D, Wodak A, Woody GE. Buprenorphine use: the international experience. Clin Infect Dis. 2006;43(Suppl 4):S197–S215. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical