Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis
- PMID: 22742601
- PMCID: PMC3843354
- DOI: 10.1111/j.1365-3016.2012.01284.x
Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis
Abstract
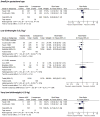
Vitamin A (VA) deficiency during pregnancy is common in low-income countries and a growing number of intervention trials have examined the effects of supplementation during pregnancy on maternal, perinatal and infant health outcomes. We systematically reviewed the literature to identify trials isolating the effects of VA or carotenoid supplementation during pregnancy on maternal, fetal, neonatal and early infant health outcomes. Meta-analysis was used to pool effect estimates for outcomes with more than one comparable study. We used GRADE criteria to assess the quality of individual studies and the level of evidence available for each outcome. We identified 23 eligible trials of which 17 had suitable quality for inclusion in meta-analyses. VA or beta-carotene (βC) supplementation during pregnancy did not have a significant overall effect on birthweight indicators, preterm birth, stillbirth, miscarriage or fetal loss. Among HIV-positive women, supplementation was protective against low birthweight (<2.5 kg) [risk ratio (RR) = 0.79 [95% confidence interval (CI) 0.64, 0.99]], but no significant effects on preterm delivery or small-for-gestational age were observed. Pooled analysis of the results of three large randomised trials found no effects of VA supplementation on neonatal/infant mortality, or pregnancy-related maternal mortality (random-effects RR = 0.86 [0.60, 1.24]) although high heterogeneity was observed in the maternal mortality estimate (I(2) = 74%, P = 0.02). VA supplementation during pregnancy was found to improve haemoglobin levels and reduce anaemia risk (<11.0 g/dL) during pregnancy (random-effects RR = 0.81 [0.69, 0.94]), also with high heterogeneity (I(2) = 52%, P = 0.04). We found no effect of VA/βC supplementation on mother-to-child HIV transmission in pooled analysis, although some evidence suggests that it may increase transmission. There is little consistent evidence of benefit of maternal supplementation with VA or βC during pregnancy on maternal or infant mortality. While there may be beneficial effects for certain outcomes, there may also be potential for harm through increased HIV transmission in some populations.
© 2012 Blackwell Publishing Ltd.
Figures





References
-
- WHO. WHO Global Database on Vitamin A deficiency. Geneva: World Health Organization; 2009. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. [cited. Available from: http://whqlibdoc.who.int/publications/2009/9789241598019_eng.pdf.
-
- West KP., Jr Extent of vitamin A deficiency among preschool children and women of reproductive age. Journal of Nutrition. 2002;132:2857S–2866S. - PubMed
-
- Christian P, West KP, Jr, Khatry SK, Katz J, LeClerq S, Pradhan EK, et al. Vitamin A or beta-carotene supplementation reduces but does not eliminate maternal night blindness in Nepal. Journal of Nutrition. 1998;128:1458–1463. - PubMed
-
- Christian P, West KP, Jr, Khatry SK, Katz J, Shrestha SR, Pradhan EK, et al. Night blindness of pregnancy in rural Nepal--nutritional and health risks. International Journal of Epidemiology. 1998;27:231–237. - PubMed
-
- Christian P, West KP, Jr, Khatry SK, LeClerq SC, Kimbrough-Pradhan E, Katz J, et al. Maternal night blindness increases risk of mortality in the first 6 months of life among infants in Nepal. Journal of Nutrition. 2001;131:1510–1512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

