Nuclear medicine imaging of gastro-entero-pancreatic neuroendocrine tumors. The key role of cellular differentiation and tumor grade: from theory to clinical practice
- PMID: 22743056
- PMCID: PMC3392783
- DOI: 10.1102/1470-7330.2012.0026
Nuclear medicine imaging of gastro-entero-pancreatic neuroendocrine tumors. The key role of cellular differentiation and tumor grade: from theory to clinical practice
Abstract
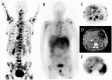
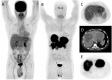
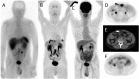
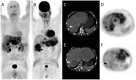
Nuclear medicine imaging is a powerful diagnostic tool for the management of patients with gastro-entero-pancreatic neuroendocrine tumors, mainly developed considering some cellular characteristics that are specific to the neuroendocrine phenotype. Hence, overexpression of specific trans membrane receptors as well as the cellular ability to take up, accumulate, and decarboxylate amine precursors have been considered for diagnostic radiotracer development. Moreover, the glycolytic metabolism, which is not a specific energetic pathway of neuroendocrine tumors, has been proposed for radionuclide imaging of neuroendocrine tumors. The results of scintigraphic examinations reflect the pathologic features and tumor metabolic properties, allowing the in vivo characterization of the disease. In this article, the influence of both cellular differentiation and tumor grade in the scintigraphic pattern is reviewed according to the literature data. The relationship between nuclear imaging results and prognosis is also discussed. Despite the existence of a relationship between the results of scintigraphic imaging and cellular differentiation, tumor grade and patient outcome, the mechanism explaining the variability of the results needs further investigation.
Figures



References
-
- Warner RR, Mani S, Profeta J, Grunstein E. Octreotide treatment of carcinoid hypertensive crisis. Mt Sinai J Med. 1994;61:349–355. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
