Ogg1 null mice exhibit age-associated loss of the nigrostriatal pathway and increased sensitivity to MPTP
- PMID: 22743193
- PMCID: PMC3468700
- DOI: 10.1016/j.neuint.2012.06.013
Ogg1 null mice exhibit age-associated loss of the nigrostriatal pathway and increased sensitivity to MPTP
Abstract
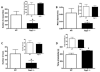
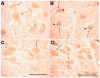
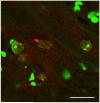
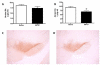
Cumulative damage to cellular macromolecules via oxidative stress is a hallmark of aging and neurodegenerative disease. Whether such damage is a cause or a subsequent effect of neurodegeneration is still unknown. This paper describes the development of an age-associated mild parkinsonian model in mice that lack the DNA repair enzyme 8-oxoguanine glycosylase 1 (Ogg1). Aged OGG1 knock-out (OGG1 KO) mice show a decreased spontaneous locomotor behavior and evidence a decrease in striatal dopamine levels, a loss of tyrosine hydroxylase (TH)-positive neurons in the substantia nigra (SN), and an increase in ubiquitin-positive inclusions in their remaining SN neurons. In addition, young OGG1 KO mice are more susceptible to the dopaminergic toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) than their wild-type (WT) counterparts. Age-associated increases in 7,8-dihydro-2'-deoxyguanine (oxo(8)dG) have been reported in brain regions and neuronal populations affected in Parkinson's disease (PD), toxin-induced parkinsonian models, and mice harboring genetic abnormalities associated with PD. Because of these increased oxo(8)dG levels, the OGG1 KO mouse strain could shed light on molecular events leading to neuronal loss as a consequence of cumulative oxidative damage to DNA during aging and after toxicological challenge.
Published by Elsevier Ltd.
Figures






References
-
- Abe T, Isobe C, Murata T, Sato C, Tohgi H. Alteration of 8-hydroxyguanosine concentrations in the cerebrospinal fluid and serum from patients with Parkinson’s disease. Neurosci Lett. 2003;336:105–108. - PubMed
-
- Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–1203. - PubMed
-
- Alves-Rodrigues A, Gregori L, Figueiredo-Pereira ME. Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci. 1998;21:516–520. - PubMed
-
- Beretta S, Wood JP, Derham B, Sala G, Tremolizzo L, Ferrarese C, Osborne NN. Partial mitochondrial complex I inhibition induces oxidative damage and perturbs glutamate transport in primary retinal cultures. Relevance to Leber Hereditary Optic Neuropathy (LHON) Neurobiol Dis. 2006;24:308–317. - PubMed
-
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

