PcrA-mediated disruption of RecA nucleoprotein filaments--essential role of the ATPase activity of RecA
- PMID: 22743269
- PMCID: PMC3458574
- DOI: 10.1093/nar/gks641
PcrA-mediated disruption of RecA nucleoprotein filaments--essential role of the ATPase activity of RecA
Abstract
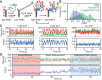
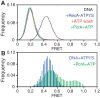
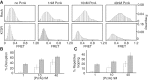
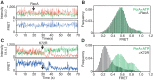
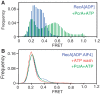
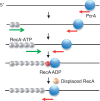
The essential DNA helicase, PcrA, regulates recombination by displacing the recombinase RecA from the DNA. The nucleotide-bound state of RecA determines the stability of its nucleoprotein filaments. Using single-molecule fluorescence approaches, we demonstrate that RecA displacement by a translocating PcrA requires the ATPase activity of the recombinase. We also show that in a 'head-on collision' between a polymerizing RecA filament and a translocating PcrA, the RecA K72R ATPase mutant, but not wild-type RecA, arrests helicase translocation. Our findings demonstrate that translocation of PcrA is not sufficient to displace RecA from the DNA and assigns an essential role for the ATPase activity of RecA in helicase-mediated disruption of its filaments.
Figures
References
-
- Lohman T, Tomko E, Wu C. Non-hexameric DNA helicases and translocases: mechanisms and regulation. Nat. Rev. Mol. Cell Biol. 2008;9:391–401. - PubMed
-
- Singleton MR, Dillingham MS, Wigley DB. Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 2007;76:23–50. - PubMed
-
- Benkovic S, Valentine A, Salinas F. Replisome-mediated DNA replication. Annu. Rev. Biochem. 2001;70:181–208. - PubMed
-
- Bidnenko V, Lestini R, Michel B. The Escherichia coli UvrD helicase is essential for Tus removal during recombination-dependent replication restart from Ter sites. Mol. Microbiol. 2006;62:382–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

