Roles of μ-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates
- PMID: 22743574
- PMCID: PMC3464037
- DOI: 10.1124/jpet.112.194308
Roles of μ-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates
Abstract
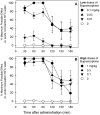
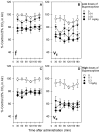
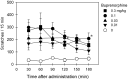
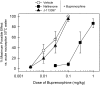
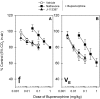
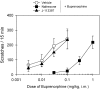
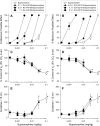
Buprenorphine is known as a μ-opioid peptide (MOP) receptor agonist, but its antinociception is compromised by the activation of nociceptin/orphanin FQ peptide (NOP) receptors in rodents. The aim of this study was to investigate the roles of MOP and NOP receptors in regulating buprenorphine-induced physiological responses in primates (rhesus monkeys). The effects of MOP antagonist (naltrexone), NOP antagonist [(±)-1-[(3R*,4R*)-1-(cyclooctylmethyl)-3-(hydroxymethyl)-4-piperidinyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one (J-113397)], and NOP agonists [(1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5] decan-4-one (Ro 64-6198) and 3-endo-8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol (SCH 221510)] on buprenorphine were studied in three functional assays for measuring analgesia, respiratory depression, and itch in primates. Over the dose range of 0.01 to 0.1 mg/kg, buprenorphine dose-dependently produced antinociception, respiratory depression, and itch/scratching responses, and there was a ceiling effect at higher doses (0.1-1 mg/kg). Naltrexone (0.03 mg/kg) produced similar degrees of rightward shifts of buprenorphine's dose-response curves for all three endpoints. Mean pK(B) values of naltrexone (8.1-8.3) confirmed that MOP receptors mediated mainly buprenorphine-induced antinociception, respiratory depression, and itch/scratching. In contrast, J-113397 (0.1 mg/kg) did not change buprenorphine-induced physiological responses, indicating that there were no functional NOP receptors in buprenorphine-induced effects. More importantly, both NOP agonists, Ro 64-6198 and SCH 221510, enhanced buprenorphine-induced antinociception without respiratory depression and itch/ scratching. The dose-addition analysis revealed that buprenorphine in combination with the NOP agonist synergistically produced antinociceptive effects. These findings provided functional evidence that the activation of NOP receptors did not attenuate buprenorphine-induced antinociception in primates; instead, the coactivation of MOP and NOP receptors produced synergistic antinociception without other side effects. This study strongly supports the therapeutic potential of mixed MOP/NOP agonists as innovative analgesics.
Figures

References
-
- Bailey PL, Rhondeau S, Schafer PG, Lu JK, Timmins BS, Foster W, Pace NL, Stanley TH. (1993) Dose-response pharmacology of intrathecal morphine in human volunteers. Anesthesiology 79:49–59; discussion 25A - PubMed
-
- Butelman ER, France CP, Woods JH. (1993) Apparent pA2 analysis on the respiratory depressant effects of alfentanil, etonitazene, ethylketocyclazocine (EKC) and Mr2033 in rhesus monkeys. J Pharmacol Exp Ther 264:145–151 - PubMed
-
- Clark MJ, Furman CA, Gilson TD, Traynor JR. (2006) Comparison of the relative efficacy and potency of μ-opioid agonists to activate Gαi/o proteins containing a pertussis toxin-insensitive mutation. J Pharmacol Exp Ther 317:858–864 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

