A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma
- PMID: 22743678
- PMCID: PMC3561510
- DOI: 10.1183/09031936.00223411
A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma
Abstract
Pre-clinical data demonstrate a pivotal role for interleukin (IL)-13 in the development and maintenance of asthma. This study assessed the effects of tralokinumab, an investigational human IL-13-neutralising immunoglobulin G4 monoclonal antibody, in adults with moderate-to-severe uncontrolled asthma despite controller therapies. 194 subjects were randomised to receive tralokinumab (150, 300 or 600 mg) or placebo subcutaneously every 2 weeks. Primary end-point was change from baseline in mean Asthma Control Questionnaire score (ACQ-6; ACQ mean of six individual item scores) at week 13 comparing placebo and combined tralokinumab dose groups. Secondary end-points included pre-bronchodilator lung function, rescue β(2)-agonist use and safety. Numerical end-points are reported as mean±sd. At week 13, change from baseline in ACQ-6 was -0.76±1.04 for tralokinumab versus -0.61±0.90 for placebo (p=0.375). Increases from baseline in forced expiratory volume in 1 s (FEV(1)) were 0.21±0.38 L versus 0.06±0.48 L (p=0.072), with a dose-response observed across the tralokinumab doses tested. β(2)-agonist use (puffs per day) was decreased for tralokinumab -0.68±1.45 versus placebo -0.10±1.49 (p=0.020). The increase in FEV(1) following tralokinumab treatment remained evident 12 weeks after the final dose. Safety profile was acceptable with no serious adverse events related to tralokinumab. No improvement in ACQ-6 was observed, although tralokinumab treatment was associated with improved lung function.
Trial registration: ClinicalTrials.gov NCT00873860.
Conflict of interest statement
Statements of interest for all authors and the study itself can be found at
Figures
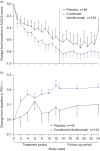

Comment in
-
Anti-interleukin-13 antibody therapy for asthma: one step closer.Eur Respir J. 2013 Feb;41(2):255-6. doi: 10.1183/09031936.00124212. Eur Respir J. 2013. PMID: 23370798 No abstract available.
References
-
- Guilbert TW, Garris C, Jhingran P, et al. Asthma that is not well-controlled is associated with increased healthcare utilization and decreased quality of life. J Asthma 2011; 48: 126–132 - PubMed
-
- Haselkorn T, Chen H, Miller DP, et al. Asthma control and activity limitations: insights from the Real-world Evaluation of Asthma Control and Treatment (REACT) study. Ann Allergy Asthma Immunol 2010; 104: 471–477 - PubMed
-
- Peters SP, Ferguson G, Deniz Y, et al. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med 2006; 100: 1139–1151 - PubMed
-
- Sullivan SD, Rasouliyan L, Russo PA, et al. Extent, patterns, and burden of uncontrolled disease in severe or difficult-to-treat asthma. Allergy 2007; 62: 126–133 - PubMed
-
- Wills-Karp M, Luyimbazi J, Xu X, et al. Interleukin-13: central mediator of allergic asthma. Science 1998; 282: 2258–2261 - PubMed
