Constructing molecular complexity and diversity: total synthesis of natural products of biological and medicinal importance
- PMID: 22743704
- PMCID: PMC3426871
- DOI: 10.1039/c2cs35116a
Constructing molecular complexity and diversity: total synthesis of natural products of biological and medicinal importance
Abstract
The advent of organic synthesis and the understanding of the molecule as they occurred in the nineteenth century and were refined in the twentieth century constitute two of the most profound scientific developments of all time. These discoveries set in motion a revolution that shaped the landscape of the molecular sciences and changed the world. Organic synthesis played a major role in this revolution through its ability to construct the molecules of the living world and others like them whose primary element is carbon. Although the early beginnings of organic synthesis came about serendipitously, organic chemists quickly recognized its potential and moved decisively to advance and exploit it in myriad ways for the benefit of mankind. Indeed, from the early days of the synthesis of urea and the construction of the first carbon-carbon bond, the art of organic synthesis improved to impressively high levels of sophistication. Through its practice, today chemists can synthesize organic molecules--natural and designed--of all types of structural motifs and for all intents and purposes. The endeavor of constructing natural products--the organic molecules of nature--is justly called both a creative art and an exact science. Often called simply total synthesis, the replication of nature's molecules in the laboratory reflects and symbolizes the state of the art of synthesis in general. In the last few decades a surge in total synthesis endeavors around the world led to a remarkable collection of achievements that covers a wide ranging landscape of molecular complexity and diversity. In this article, we present highlights of some of our contributions in the field of total synthesis of natural products of biological and medicinal importance. For perspective, we also provide a listing of selected examples of additional natural products synthesized in other laboratories around the world over the last few years.
Figures

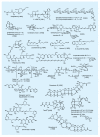
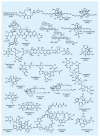
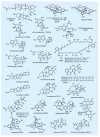
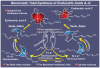
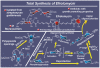










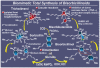
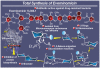


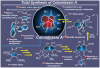
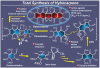
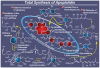
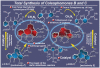

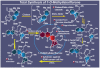
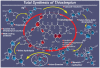
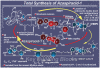
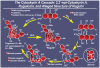
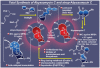

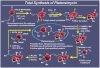
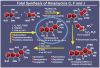
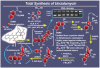
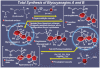
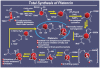
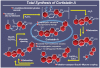
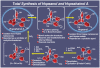
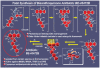
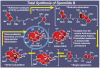
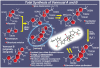
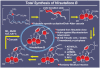
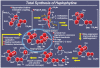
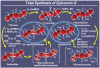

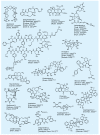
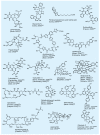
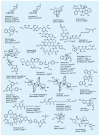
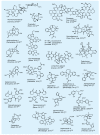
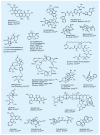
References
-
- Nicolaou KC, Montangon T. Molecules That Changed the World. Wiley-VCH Publishers; Weinheim: 2008. p. 366.
-
- Rocke AJ. Image and Reality: Kekulé, Kopp, and the Scientific Imagination. The University of Chicago Press; Chicago: 2010. p. 375.
- Levere TH. Transforming Matter. Johns Hopkins Univesity Press; 2001. p. 215.
-
- Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. VCH Publishers; Weinheim, Germany; 1996. p. 798.
-
- Nicolaou KC, Snyder SA. Classics in Total Synthesis II. Wiley-VCH Publishers; Weinheim, Germany: 2003. p. 636.
-
- Nicolaou KC, Chen JS. Classics in Total Synthesis III. Wiley-VCH Publishers; Weinheim, Germany: 2011. p. 746.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

