Protein misfolding cyclic amplification of infectious prions
- PMID: 22743831
- PMCID: PMC4049227
- DOI: 10.1038/nprot.2012.067
Protein misfolding cyclic amplification of infectious prions
Abstract
Prions are proteinaceous infectious agents responsible for the transmission of prion diseases. The lack of a procedure for cultivating prions in the laboratory has been a major limitation to the study of the unorthodox nature of this infectious agent and the molecular mechanism by which the normal prion protein (PrP(C)) is converted into the abnormal isoform (PrP(Sc)). Protein misfolding cyclic amplification (PMCA), described in detail in this protocol, is a simple, fast and efficient methodology to mimic prion replication in the test tube. PMCA involves incubating materials containing minute amounts of infectious prions with an excess of PrP(C) and boosting the conversion by cycles of sonication to fragment the converting units, thereby leading to accelerated prion replication. PMCA is able to detect the equivalent of a single molecule of infectious PrP(Sc) and propagate prions that maintain high infectivity, strain properties and species specificity. A single PMCA assay takes little more than 3 d to replicate a large amount of prions, which could take years in an in vivo situation. Since its invention 10 years ago, PMCA has helped to answer fundamental questions about this intriguing infectious agent and has been broadly applied in research areas that include the food industry, blood bank safety and human and veterinary disease diagnosis.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures

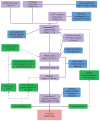

References
-
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. - PubMed
-
- Collee JG, Bradley R. BSE: a decade on—Part I. Lancet. 1997;349:636–641. - PubMed
-
- Collee JG, Bradley R. BSE: a decade on—Part 2. Lancet. 1997;349:715–721. - PubMed
-
- Llewelyn CA, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

