Unveiling an exceptional zymogen: the single-chain form of tPA is a selective activator of NMDA receptor-dependent signaling and neurotoxicity
- PMID: 22743997
- PMCID: PMC3504712
- DOI: 10.1038/cdd.2012.86
Unveiling an exceptional zymogen: the single-chain form of tPA is a selective activator of NMDA receptor-dependent signaling and neurotoxicity
Abstract
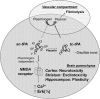
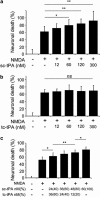
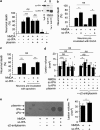
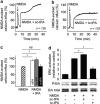
Unlike other serine proteases that are zymogens, the single-chain form of tissue plasminogen activator (sc-tPA) exhibits an intrinsic activity similar to that of its cleaved two-chain form (tc-tPA), especially in the presence of fibrin. In the central nervous system tPA controls brain functions and dysfunctions through its proteolytic activity. We demonstrated here, both in vitro and in vivo, that the intrinsic activity of sc-tPA selectively modulates N-methyl-D-aspartate receptor (NMDAR) signaling as compared with tc-tPA. Thus, sc-tPA enhances NMDAR-mediated calcium influx, Erk(½) activation and neurotoxicity in cultured cortical neurons, excitotoxicity in the striatum and NMDAR-dependent long-term potentiation in the hippocampal CA-1 network. As the first demonstration of a differential function for sc-tPA and tc-tPA, this finding opens a new area of investigations on tPA functions in the absence of its allosteric regulator, fibrin.
Figures




Similar articles
-
The functional role of the second NPXY motif of the LRP1 beta-chain in tissue-type plasminogen activator-mediated activation of N-methyl-D-aspartate receptors.J Biol Chem. 2008 May 2;283(18):12004-13. doi: 10.1074/jbc.M707607200. Epub 2008 Mar 5. J Biol Chem. 2008. PMID: 18321860
-
Conformations of tissue plasminogen activator (tPA) orchestrate neuronal survival by a crosstalk between EGFR and NMDAR.Cell Death Dis. 2015 Oct 15;6(10):e1924. doi: 10.1038/cddis.2015.296. Cell Death Dis. 2015. PMID: 26469972 Free PMC article.
-
NR2D-containing NMDA receptors mediate tissue plasminogen activator-promoted neuronal excitotoxicity.Cell Death Differ. 2010 May;17(5):860-71. doi: 10.1038/cdd.2009.172. Epub 2009 Nov 13. Cell Death Differ. 2010. PMID: 19911010
-
Tissue-type plasminogen activator controls neuronal death by raising surface dynamics of extrasynaptic NMDA receptors.Cell Death Dis. 2016 Nov 10;7(11):e2466. doi: 10.1038/cddis.2016.279. Cell Death Dis. 2016. PMID: 27831563 Free PMC article.
-
Tissue plasminogen activator in the amygdala: a new role for an old protease.J Physiol Pharmacol. 2008 Dec;59 Suppl 8:135-46. J Physiol Pharmacol. 2008. PMID: 19258671 Review.
Cited by
-
Neuroserpin Differentiates Between Forms of Tissue Type Plasminogen Activator via pH Dependent Deacylation.Front Cell Neurosci. 2016 Jun 15;10:154. doi: 10.3389/fncel.2016.00154. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27378851 Free PMC article.
-
Identification of a neurovascular signaling pathway regulating seizures in mice.Ann Clin Transl Neurol. 2015 Jul;2(7):722-38. doi: 10.1002/acn3.209. Epub 2015 May 1. Ann Clin Transl Neurol. 2015. PMID: 26273685 Free PMC article.
-
Two-Chains Tissue Plasminogen Activator Unifies Met and NMDA Receptor Signalling to Control Neuronal Survival.Int J Mol Sci. 2021 Dec 15;22(24):13483. doi: 10.3390/ijms222413483. Int J Mol Sci. 2021. PMID: 34948279 Free PMC article.
-
Tissue plasminogen activator inhibits NMDA-receptor-mediated increases in calcium levels in cultured hippocampal neurons.Front Cell Neurosci. 2015 Oct 9;9:404. doi: 10.3389/fncel.2015.00404. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26500501 Free PMC article.
-
Impacts of tissue-type plasminogen activator (tPA) on neuronal survival.Front Cell Neurosci. 2015 Oct 16;9:415. doi: 10.3389/fncel.2015.00415. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26528141 Free PMC article. Review.
References
-
- Angles-Cano E, Balaton A, Le Bonniec B, Genot E, Elion J, Sultan Y. Production of monoclonal antibodies to the high fibrin-affinity, tissue-type plasminogen activator of human plasma. Demonstration of its endothelial origin by immunolocalization. Blood. 1985;66:913–920. - PubMed
-
- Rijken DC, Hoylaerts M, Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem. 1982;257:2920–2925. - PubMed
-
- Rajapakse S, Ogiwara K, Takano N, Moriyama A, Takahashi T. Biochemical characterization of human kallikrein 8 and its possible involvement in the degradation of extracellular matrix proteins. FEBS Lett. 2005;579:6879–6884. - PubMed
-
- Thelwell C, Longstaff C. The regulation by fibrinogen and fibrin of tissue plasminogen activator kinetics and inhibition by plasminogen activator inhibitor 1. J Thromb Haemost. 2007;5:804–811. - PubMed
-
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

