Adverse drug reaction: rosuvastatin as a cause for ischaemic colitis in a 64-year-old woman
- PMID: 22744258
- PMCID: PMC3448351
- DOI: 10.1136/bcr.11.2011.5270
Adverse drug reaction: rosuvastatin as a cause for ischaemic colitis in a 64-year-old woman
Abstract
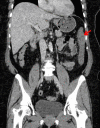
Rosuvastatin (Crestor, AstraZeneca) is a commonly used drug for managing hypercholesterolaemia. It is a very safe medication with mostly acceptable side effects. Rare but serious side effects are not well known. A 64-year-old woman presented with bloody diarrhoea after starting rosuvastatin for hypercholesterolaemia. Stool microscopy and culture ruled out infective causes. Abdominal CT scan revealed normal calibre celiac axis and superior mesenteric artery. Colonoscopic biopsy revealed ischaemic colitis as the final histological diagnosis. The patient is in complete remission after ceasing the medication. Rosuvastatin causing ischaemic colitis should be considered a rare but serious adverse drug reaction.
Conflict of interest statement
Figures


References
-
- Hsia J, MacFadyen JG, Monyak J, et al. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin: the JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol 2011;57:1666–75. - PubMed
-
- Kostapanos MS, Milionis HJ, Elisaf MS. Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia. Am J Cardiovasc Drugs 2010;10:11–28. - PubMed
-
- “FDA Approves New Drug for Lowering Cholesterol”. The Food and Drug Administration. August 12, 2003.
-
- Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis. The ASTEROID trial. JAMA 2006;295:1556–65. - PubMed
-
- Micromedex. http://www.micromedex.com/mobile/ (accessed 7 September 2011).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
