Interferon regulatory factor-2 is protective against hepatic ischemia-reperfusion injury
- PMID: 22744333
- PMCID: PMC3468551
- DOI: 10.1152/ajpgi.00050.2012
Interferon regulatory factor-2 is protective against hepatic ischemia-reperfusion injury
Abstract
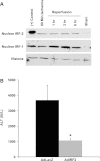
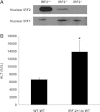
Interferon regulatory factor (IRF)-1 is a nuclear transcription factor that induces inflammatory cytokine mediators and contributes to hepatic ischemia-reperfusion (I/R) injury. No strategies to mitigate IRF1-mediated liver damage exist. IRF2 is a structurally similar endogenous protein that competes with IRF1 for DNA binding sites in IRF-responsive target genes and acts as a competitive inhibitor. However, the role of IRF2 in hepatic injury during hypoxic or inflammatory conditions is unknown. We hypothesize that IRF2 overexpression may mitigate IRF1-mediated I/R damage. Endogenous IRF2 is basally expressed in normal livers and is mildly increased by ischemia alone. Overexpression of IRF2 protects against hepatic warm I/R injury. Furthermore, we demonstrate that IRF2 overexpression limits production of IRF1-dependent proinflammatory genes, such as IL-12, IFNβ, and inducible nitric oxide synthase, even in the presence of IRF1 induction. Additionally, isograft liver transplantation with IRF2 heterozygote knockout (IRF2(+/-)) donor grafts that have reduced endogenous IRF2 levels results in worse injury following cold I/R during murine orthotopic liver transplantation. These findings indicate that endogenous intrahepatic IRF2 protein is protective, because the IRF2-deficient liver donor grafts exhibited increased liver damage compared with the wild-type donor grafts. In summary, IRF2 overexpression protects against I/R injury by decreasing IRF1-dependent injury and may represent a novel therapeutic strategy.
Figures




References
-
- Cuesta N, Nhu QM, Zudaire E, Polumuri S, Cuttitta F, Vogel SN. IFN regulatory factor-2 regulates macrophage apoptosis through a STAT1/3- and caspase-1-dependent mechanism. J Immunol 178: 3602–3611, 2007 - PubMed
-
- Cuesta N, Salkowski CA, Thomas KE, Vogel SN. Regulation of lipopolysaccharide sensitivity by IFN regulatory factor-2. J Immunol 170: 5739–5747, 2003 - PubMed
-
- Fujita T, Kimura Y, Miyamoto M, Barsoumian EL, Taniguchi T. Induction of endogenous IFN-α and IFN-β genes by a regulatory transcription factor, IRF-1. Nature 337: 270–272, 1989 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

