Protein kinase Cδ differentially regulates cAMP-dependent translocation of NTCP and MRP2 to the plasma membrane
- PMID: 22744337
- PMCID: PMC3468552
- DOI: 10.1152/ajpgi.00529.2011
Protein kinase Cδ differentially regulates cAMP-dependent translocation of NTCP and MRP2 to the plasma membrane
Abstract
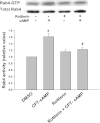
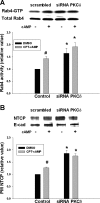
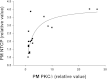
Cyclic AMP stimulates translocation of Na(+)/taurocholate cotransporting polypeptide (NTCP) from the cytosol to the sinusoidal membrane and multidrug resistance-associated protein 2 (MRP2) to the canalicular membrane. A recent study suggested that protein kinase Cδ (PKCδ) may mediate cAMP-induced translocation of Ntcp and Mrp2. In addition, cAMP has been shown to stimulate NTCP translocation in part via Rab4. The aim of this study was to determine whether cAMP-induced translocation of NTCP and MRP2 require kinase activity of PKCδ and to test the hypothesis that cAMP-induced activation of Rab4 is mediated via PKCδ. Studies were conducted in HuH-NTCP cells (HuH-7 cells stably transfected with NTCP). Transfection of cells with wild-type PKCδ increased plasma membrane PKCδ and NTCP and increased Rab4 activity. Paradoxically, overexpression of kinase-dead dominant-negative PKCδ also increased plasma membrane PKCδ and NTCP as well as Rab4 activity. Similar results were obtained in PKCδ knockdown experiments, despite a decrease in total PKCδ. These results raised the possibility that plasma membrane localization rather than kinase activity of PKCδ is necessary for NTCP translocation and Rab4 activity. This hypothesis was supported by results showing that rottlerin, which has previously been shown to inhibit cAMP-induced membrane translocation of PKCδ and NTCP, inhibited cAMP-induced Rab4 activity. In addition, LY294002 (a phosphoinositide-3-kinase inhibitor), which has been shown to inhibit cAMP-induced NTCP translocation, also inhibited cAMP-induced PKCδ translocation. In contrast to the results with NTCP, cAMP-induced MRP2 translocation was inhibited in cells transfected with DN-PKCδ and small interfering RNA PKCδ. Taken together, these results suggest that the plasma membrane localization rather than kinase activity of PKCδ plays an important role in cAMP-induced NTCP translocation and Rab4 activity, whereas the kinase activity of PKCδ is necessary for cAMP-induced MRP2 translocation.
Figures







References
-
- Anwer MS. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology 39: 581–590, 2004 - PubMed
-
- Bailly E, McCaffrey M, Touchot N, Zahraoui A, Goud B, Bornens M. Phosphorylation of two small GTP-binding proteins of the Rab family by p34cdc2. Nature 350: 715–718, 1991 - PubMed
-
- Becker KP, Hannun YA. cPKC-dependent sequestration of membrane-recycling components in a subset of recycling endosomes. J Biol Chem 278: 52747–52754, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

