The Drosophila neurally altered carbohydrate mutant has a defective Golgi GDP-fucose transporter
- PMID: 22745127
- PMCID: PMC3436151
- DOI: 10.1074/jbc.M112.379313
The Drosophila neurally altered carbohydrate mutant has a defective Golgi GDP-fucose transporter
Abstract
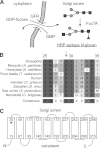
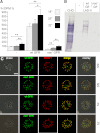
Studying genetic disorders in model organisms can provide insights into heritable human diseases. The Drosophila neurally altered carbohydrate (nac) mutant is deficient for neural expression of the HRP epitope, which consists of N-glycans with core α1,3-linked fucose residues. Here, we show that a conserved serine residue in the Golgi GDP-fucose transporter (GFR) is substituted by leucine in nac(1) flies, which abolishes GDP-fucose transport in vivo and in vitro. This loss of function is due to a biochemical defect, not to destabilization or mistargeting of the mutant GFR protein. Mass spectrometry and HPLC analysis showed that nac(1) mutants lack not only core α1,3-linked, but also core α1,6-linked fucose residues on their N-glycans. Thus, the nac(1) Gfr mutation produces a previously unrecognized general defect in N-glycan core fucosylation. Transgenic expression of a wild-type Gfr gene restored the HRP epitope in neural tissues, directly demonstrating that the Gfr mutation is solely responsible for the neural HRP epitope deficiency in the nac(1) mutant. These results validate the Drosophila nac(1) mutant as a model for the human congenital disorder of glycosylation, CDG-IIc (also known as LAD-II), which is also the result of a GFR deficiency.
Figures



References
-
- Morava E., Lefeber D. J., Wevers R. A. (2011) Protein glysosylation and congenital disorders of glycosylation. in Post-translational Modifications in Health and Disease (Vidal C. J., ed) pp. 97–117, Springer, New York
-
- Jaeken J. (2010) Congenital disorders of glycosylation. Ann. N.Y. Acad. Sci. 1214, 190–198 - PubMed
-
- Fukuda M. N., Papayannopoulou T., Gordon-Smith E. C., Rochant H., Testa U. (1984) Defect in glycosylation of erythrocyte membrane proteins in congenital dyserythropoietic anemia type II (HEMPAS). Br. J. Haematol. 56, 55–68 - PubMed
-
- Jaeken J., van Eijk H. G., van der Heul C., Corbeel L., Eeckels R., Eggermont E. (1984) Sialic acid-deficient serum and cerebrospinal fluid transferrin in a newly recognized genetic syndrome. Clin. Chim. Acta 144, 245–247 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

