Evidence of the importance of the first intracellular loop of prokineticin receptor 2 in receptor function
- PMID: 22745195
- PMCID: PMC3404297
- DOI: 10.1210/me.2012-1102
Evidence of the importance of the first intracellular loop of prokineticin receptor 2 in receptor function
Abstract
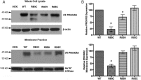
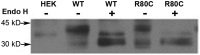
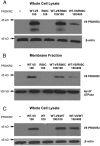
Prokineticin receptors (PROKR) are G protein-coupled receptors (GPCR) that regulate diverse biological processes, including olfactory bulb neurogenesis and GnRH neuronal migration. Mutations in PROKR2 have been described in patients with varying degrees of GnRH deficiency and are located in diverse functional domains of the receptor. Our goal was to determine whether variants in the first intracellular loop (ICL1) of PROKR2 (R80C, R85C, and R85H) identified in patients with hypogonadotropic hypogonadism interfere with receptor function and to elucidate the mechanisms of these effects. Because of structural homology among GPCR, clarification of the role of ICL1 in PROKR2 activity may contribute to a better understanding of this domain across other GPCR. The effects of the ICL1 PROKR2 mutations on activation of signal transduction pathways, ligand binding, and receptor expression were evaluated. Our results indicated that the R85C and R85H PROKR2 mutations interfere only modestly with receptor function, whereas the R80C PROKR2 mutation leads to a marked reduction in receptor activity. Cotransfection of wild-type (WT) and R80C PROKR2 showed that the R80C mutant could exert a dominant negative effect on WT PROKR2 in vitro by interfering with WT receptor expression. In summary, we have shown the importance of Arg80 in ICL1 for PROKR2 expression and demonstrate that R80C PROKR2 exerts a dominant negative effect on WT PROKR2.
Figures



References
-
- Ngan ES, Tam PK. 2008. Prokineticin-signaling pathway. Int J Biochem Cell Biol 40:1679–1684 - PubMed
-
- Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. 2002. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem 277:19276–19280 - PubMed
-
- Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K. 2002. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta 1579:173–179 - PubMed
-
- Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, Yamada T, Hinuma S, Inatomi N, Ohtaki T, Onda H, Fujino M. 2002. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun 293:396–402 - PubMed
-
- Chen J, Kuei C, Sutton S, Wilson S, Yu J, Kamme F, Mazur C, Lovenberg T, Liu C. 2005. Identification and pharmacological characterization of prokineticin 2β as a selective ligand for prokineticin receptor 1. Mol Pharmacol 67:2070–2076 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

