The radiological spectrum of pulmonary lymphoproliferative disease
- PMID: 22745203
- PMCID: PMC3474050
- DOI: 10.1259/bjr/16420165
The radiological spectrum of pulmonary lymphoproliferative disease
Abstract
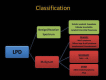
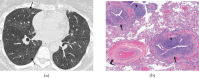
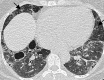
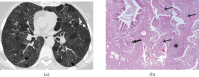
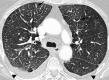
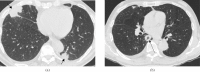
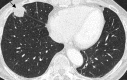
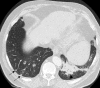
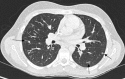
Pulmonary lymphoproliferative disorders (LPD) are characterised by abnormal proliferation of indigenous cell lines or infiltration of lung parenchyma by lymphoid cells. They encompass a wide spectrum of focal or diffuse abnormalities, which may be classified as reactive or neoplastic on the basis of cellular morphology and clonality. The spectrum of reactive disorders results primarily from antigenic stimulation of bronchial mucosa-associated lymphoid tissue (MALT) and comprises three main entities: follicular bronchiolitis, lymphoid interstitial pneumonia and (more rarely) nodular lymphoid hyperplasia. Primary parenchymal neoplasms are most commonly extranodal marginal zone lymphomas of MALT origin (MALT lymphomas), followed by diffuse large B-cell lymphomas (DLBCLs) and lymphomatoid granulomatosis (LYG). Secondary lymphomatous parenchymal neoplasms (both Hodgkin and non-Hodgkin lymphomas) are far more prevalent than primary neoplasms. Acquired immune deficiency syndrome (AIDS)-related lymphoma (ARL) and post-transplantation lymphoproliferative disorder (PTLD) may also primarily affect the lung parenchyma. Modern advances in treatments for AIDS and transplant medicine are associated with an increase in the incidence of LPD and have heightened the need to understand the range of imaging appearance of these diseases. The multidetector CT (MDCT) findings of LPD are heterogeneous, thereby reflecting the wide spectrum of clinical manifestations of these entities. Understanding the spectrum of LPD and the various imaging manifestations is crucial because the radiologist is often the first one to suggest the diagnosis and has a pivotal role in differentiating these diseases. The current concepts of LPD are discussed together with a demonstration of the breadth of MDCT patterns within this disease spectrum.
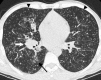
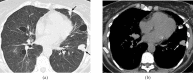
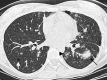
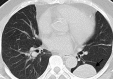
Figures

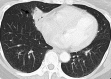
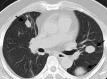
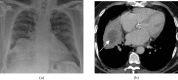
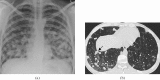
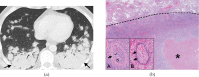
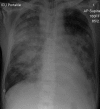
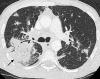
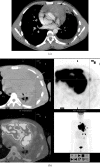
References
-
- Bienenstock J, Johnston N, Perey DY. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest 1973;28:686–92 - PubMed
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. , eds WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edn. Lyon, France: IARC; 2008
-
- Berkman N, Breuer R, Kramer MR, Polliack A. Pulmonary involvement in lymphoma. Leuk Lymphoma 1996;20:229–37 - PubMed
-
- Saltzstein SL. Pulmonary malignant lymphomas and pseudolymphomas: classification, therapy, and prognosis. Cancer 1963;16:928–55 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

