Synaptic vesicle pools and dynamics
- PMID: 22745285
- PMCID: PMC3405865
- DOI: 10.1101/cshperspect.a013680
Synaptic vesicle pools and dynamics
Abstract
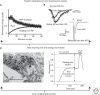
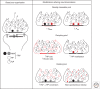
Synaptic vesicles release neurotransmitter at chemical synapses, thus initiating the flow of information in neural networks. To achieve this, vesicles undergo a dynamic cycle of fusion and retrieval to maintain the structural and functional integrity of the presynaptic terminals in which they reside. Moreover, compelling evidence indicates these vesicles differ in their availability for release and mobilization in response to stimuli, prompting classification into at least three different functional pools. Ongoing studies of the molecular and cellular bases for this heterogeneity attempt to link structure to physiology and clarify how regulation of vesicle pools influences synaptic strength and presynaptic plasticity. We discuss prevailing perspectives on vesicle pools, the role they play in shaping synaptic transmission, and the open questions that challenge current understanding.
Figures

References
-
- Abbott LF, Regehr WG 2004. Synaptic computation. Nature 431: 796–803 - PubMed
-
- Abbott LF, Varela JA, Sen K, Nelson SB 1997. Synaptic depression and cortical gain control. Science 275: 220–224 - PubMed
-
- Ahmari SE, Buchanan J, Smith SJ 2000. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci 3: 445–451 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
