Concerted control of gliogenesis by InR/TOR and FGF signalling in the Drosophila post-embryonic brain
- PMID: 22745312
- PMCID: PMC3392704
- DOI: 10.1242/dev.074179
Concerted control of gliogenesis by InR/TOR and FGF signalling in the Drosophila post-embryonic brain
Abstract
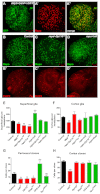
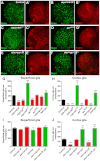
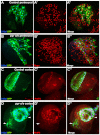
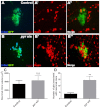
Glial cells are essential for the development and function of the nervous system. In the mammalian brain, vast numbers of glia of several different functional types are generated during late embryonic and early foetal development. However, the molecular cues that instruct gliogenesis and determine glial cell type are poorly understood. During post-embryonic development, the number of glia in the Drosophila larval brain increases dramatically, potentially providing a powerful model for understanding gliogenesis. Using glial-specific clonal analysis we find that perineural glia and cortex glia proliferate extensively through symmetric cell division in the post-embryonic brain. Using pan-glial inhibition and loss-of-function clonal analysis we find that Insulin-like receptor (InR)/Target of rapamycin (TOR) signalling is required for the proliferation of perineural glia. Fibroblast growth factor (FGF) signalling is also required for perineural glia proliferation and acts synergistically with the InR/TOR pathway. Cortex glia require InR in part, but not downstream components of the TOR pathway, for proliferation. Moreover, cortex glia absolutely require FGF signalling, such that inhibition of the FGF pathway almost completely blocks the generation of cortex glia. Neuronal expression of the FGF receptor ligand Pyramus is also required for the generation of cortex glia, suggesting a mechanism whereby neuronal FGF expression coordinates neurogenesis and cortex gliogenesis. In summary, we have identified two major pathways that control perineural and cortex gliogenesis in the post-embryonic brain and have shown that the molecular circuitry required is lineage specific.
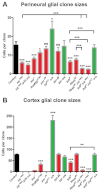
Figures



References
-
- Bateman J. M., McNeill H. (2004). Temporal control of differentiation by the insulin receptor/tor pathway in Drosophila. Cell 119, 87-96 - PubMed
-
- Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E. (2001). An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11, 213-221 - PubMed
-
- Chahrour M., Zoghbi H. Y. (2007). The story of Rett syndrome: from clinic to neurobiology. Neuron 56, 422-437 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

