Simulated GABA synaptic input and L-type calcium channels form functional microdomains in hypothalamic gonadotropin-releasing hormone neurons
- PMID: 22745478
- PMCID: PMC3401604
- DOI: 10.1523/JNEUROSCI.4188-11.2012
Simulated GABA synaptic input and L-type calcium channels form functional microdomains in hypothalamic gonadotropin-releasing hormone neurons
Abstract
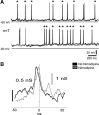
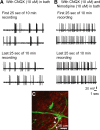
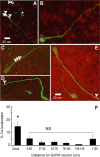
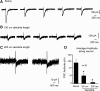
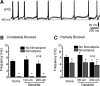
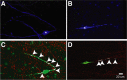
Hypothalamic gonadotropin-releasing hormone (GnRH) neurons integrate the multiple internal and external cues that regulate sexual reproduction. In contrast to other neurons that exhibit extensive dendritic arbors, GnRH neurons usually have a single dendrite with relatively little branching. This largely precludes the integration strategy in which a single dendritic branch serves as a unit of integration. In the present study, we identify a gradient in L-type calcium channels in dendrites of mouse GnRH neurons and its interaction with GABAergic and glutamatergic inputs. Higher levels of L-type calcium channels are in somata/proximal dendrites (i.e., 0-26 μm) and distal dendrites (∼130 μm dendrite length), but intervening midlengths of dendrite (∼27-130 μm) have reduced L-type calcium channels. Using uncaging of GABA, there is a decreasing GABAergic influence along the dendrite and the impact of GABA(A) receptors is dependent on activation of L-type calcium channels. This results in amplification of proximal GABAergic signals and attenuation of distal dendritic signals. Most interestingly, the intervening dendritic regions create a filter through which only relatively high-amplitude, low-frequency GABAergic signaling to dendrites elicits action potentials. The findings of the present study suggest that GnRH dendrites adopt an integration strategy whereby segments of single nonbranching GnRH dendrites create functional microdomains and thus serve as units of integration.
Figures


Similar articles
-
Synaptic integration in hypothalamic gonadotropin releasing hormone (GnRH) neurons.Neuroscience. 2008 Jul 17;154(4):1337-51. doi: 10.1016/j.neuroscience.2008.04.067. Epub 2008 May 9. Neuroscience. 2008. PMID: 18556136 Free PMC article.
-
Spatially selective, testosterone-independent remodeling of dendrites in gonadotropin-releasing hormone (GnRH) neurons prepubertally in male rats.Endocrinology. 2011 May;152(5):2011-9. doi: 10.1210/en.2010-0871. Epub 2011 Feb 22. Endocrinology. 2011. PMID: 21343259 Free PMC article.
-
Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability.J Neurosci. 2011 Feb 16;31(7):2421-30. doi: 10.1523/JNEUROSCI.5759-10.2011. J Neurosci. 2011. PMID: 21325509 Free PMC article.
-
Redefining the gonadotrophin-releasing hormone neurone dendrite.J Neuroendocrinol. 2010 Jul;22(7):650-8. doi: 10.1111/j.1365-2826.2010.02032.x. Epub 2010 May 18. J Neuroendocrinol. 2010. PMID: 20492365 Review.
-
Understanding calcium homeostasis in postnatal gonadotropin-releasing hormone neurons using cell-specific Pericam transgenics.Cell Calcium. 2012 Mar-Apr;51(3-4):267-76. doi: 10.1016/j.ceca.2011.11.005. Epub 2011 Dec 15. Cell Calcium. 2012. PMID: 22177387 Review.
Cited by
-
Chloride Accumulators NKCC1 and AE2 in Mouse GnRH Neurons: Implications for GABAA Mediated Excitation.PLoS One. 2015 Jun 25;10(6):e0131076. doi: 10.1371/journal.pone.0131076. eCollection 2015. PLoS One. 2015. PMID: 26110920 Free PMC article.
-
Meta-Analysis of Heifer Traits Identified Reproductive Pathways in Bos indicus Cattle.Genes (Basel). 2021 May 18;12(5):768. doi: 10.3390/genes12050768. Genes (Basel). 2021. PMID: 34069992 Free PMC article.
-
In vivo recordings of GnRH neuron firing reveal heterogeneity and dependence upon GABAA receptor signaling.J Neurosci. 2013 May 29;33(22):9394-401. doi: 10.1523/JNEUROSCI.0533-13.2013. J Neurosci. 2013. PMID: 23719807 Free PMC article.
-
A unified model for two modes of bursting in GnRH neurons.J Comput Neurosci. 2016 Jun;40(3):297-315. doi: 10.1007/s10827-016-0598-4. Epub 2016 Mar 15. J Comput Neurosci. 2016. PMID: 26975615 Free PMC article.
-
GPR30 disrupts the balance of GABAergic and glutamatergic transmission in the spinal cord driving to the development of bone cancer pain.Oncotarget. 2016 Nov 8;7(45):73462-73472. doi: 10.18632/oncotarget.11867. Oncotarget. 2016. PMID: 27608844 Free PMC article.
References
-
- Branco T, Häusser M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol. 2010;20:494–502. - PubMed
-
- Campbell RE, Han SK, Herbison AE. Biocytin filling of adult gonadotropin-releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology. 2005;146:1163–1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
