Nickel(II) complexes stabilized by bis[N-(6-pivalamido-2-pyridylmethyl)]benzylamine: Synthesis and characterization of complexes stabilized by a hydrogen bonding network
- PMID: 22745511
- PMCID: PMC3382998
- DOI: 10.1016/j.ica.2010.05.006
Nickel(II) complexes stabilized by bis[N-(6-pivalamido-2-pyridylmethyl)]benzylamine: Synthesis and characterization of complexes stabilized by a hydrogen bonding network
Abstract
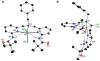
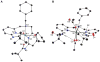
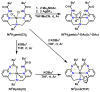
Hydrogen bonds in metalloproteins are key in directing reactivity yet to be achieved in synthetic systems. We have been developing a synthetic system that uses hydrogen-bonding interactions to modulate the secondary coordination around a transition metal ion. This was accomplished with the ligand bis[N-(6-pivalamido-2-pyridylmethyl)]benzylamine (H(2)pmb), which contains two carboxyamido units appended from pyridine rings. Several nickel complexes were prepared and structurally characterized. In particular, we found that the appended carboxyamido groups either provide intramolecular H-bond donors or can be converted to bind directly to a metal center. We established that the complex Ni(II)H(2)pmb(Cl)(2) can be sequentially deprotonated with potassium tert-butoxide, causing coordination of the carboxyamido oxygen atoms and concomitant loss of the chloro ligands. The chloro ligands were also removed with silver(I) salts-in the presence of acetate ions, the complex Ni(II)H(2)pmb(κ(2)-OAc)(κ(1)-OAc) was isolated, in which an intramolecular H-bonding network occurs between the H(2)pmb ligand and the coordinate acetato ligands.
Figures
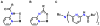
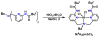
Similar articles
-
A modular approach toward regulating the secondary coordination sphere of metal ions: differential dioxygen activation assisted by intramolecular hydrogen bonds.J Am Chem Soc. 2006 Dec 6;128(48):15476-89. doi: 10.1021/ja063935+. J Am Chem Soc. 2006. PMID: 17132015
-
Molecular designs for controlling the local environments around metal ions.Acc Chem Res. 2015 Aug 18;48(8):2407-14. doi: 10.1021/acs.accounts.5b00212. Epub 2015 Jul 16. Acc Chem Res. 2015. PMID: 26181849 Free PMC article.
-
Structural diversity in manganese, iron and cobalt complexes of the ditopic 1,2-bis(2,2'-bipyridyl-6-yl)ethyne ligand and observation of epoxidation and catalase activity of manganese compounds.Dalton Trans. 2010 Aug 21;39(31):7266-75. doi: 10.1039/b925129d. Epub 2010 Jun 25. Dalton Trans. 2010. PMID: 20582360
-
Ni(II)/H(2)O(2) reactivity in bis[(pyridin-2-yl)methyl]amine tridentate ligand system. aromatic hydroxylation reaction by bis(mu-oxo)dinickel(III) complex.Inorg Chem. 2009 Jun 1;48(11):4997-5004. doi: 10.1021/ic900059m. Inorg Chem. 2009. PMID: 19374371
-
Complexes of 2,6-bis[N-(2'-pyridylmethyl)carbamyl]pyridine: formation of mononuclear complexes, and self-assembly of double helical dinuclear and tetranuclear copper(II) and trinuclear nickel(II) complexes.Dalton Trans. 2005 Feb 7;(3):518-27. doi: 10.1039/b414251a. Epub 2005 Jan 4. Dalton Trans. 2005. PMID: 15672196
Cited by
-
Structural diversity in metal complexes with a dinucleating ligand containing carboxyamidopyridyl groups.Inorg Chem. 2011 Sep 5;50(17):7922-4. doi: 10.1021/ic200881t. Epub 2011 Jul 27. Inorg Chem. 2011. PMID: 21793511 Free PMC article.
References
-
- Lu Y, Valentine JS. Curr Opin Struct Biol. 1997;7:495–500. - PubMed
- Natale D, Marque-Rivas JC. Chem Comm. 2008:425–437. - PubMed
- Marque-Rivas JC. Curr Org Chem. 2007;11:1434–1449.
- Steiner T. Angew Chem Int Ed. 2002;41:48–76. - PubMed
- Miller AF. Acc Chem Res. 2008;41:501–510. - PubMed
- Jackson TA, Brunold TC. Acc Chem Res. 2004;37:461–470. - PubMed
-
-
Representative examples: Lipscomb WN, Strater N. Chem Rev. 1996;96:2375–2433.Bhaumik D, Medin J, Gathy K, Coleman MS. J Biol Chem. 1993;268:5464–5470.Sideraki V, Mihamedali KA, Wilson DK, Chang Z, Kellems RE, Quiocho FA, Rudolph FB. Biochemistry. 1996;35:7862–7872.
-
-
-
Reviews: Shook RL, Borovik AS. Chem Comm. 2008:6095–6107.Borovik AS. Acc Chem Res. 2005;38:54–61.Marque-Rivas JC, Hinchley SL, Metteau L, Parsons S. Dalton Trans. 2006:2316–2322.
-
-
-
Harata M, Hasegawa K, Jitsukawa K, Masuda H, Einaga H. Bull Chem Soc, Jpn. 1998;71:1031–1038.Harata M, Jitsukawa J, Masuda H, Einage H. J Am Chem Soc. 1994;116:10817–10819.Berreau LM, Mahapatra S, Halfen JA, Young VG, Jr, Tolman WB. Inorg, Chem. 1996;35:6339–6342.
-
-
-
Mareque-Rivas JC, Salvagni E, Parsons S. Dalton Trans. 2004:4185–4192.Natale D, Mareque-Rivas JC. Chem Commun. 2008:425–437.Mareques Rivas JC, de Rosales RTM, Parsons S. Dalton Trans. 2003:2156–2163.Mareques Rivas JC, Salvagni E, de Rosales RTM, Parsons S. Dalton Trans. 2003:3339–3349.(e)
-
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
