Particokinetics: computational analysis of the superparamagnetic iron oxide nanoparticles deposition process
- PMID: 22745539
- PMCID: PMC3383311
- DOI: 10.2147/IJN.S30074
Particokinetics: computational analysis of the superparamagnetic iron oxide nanoparticles deposition process
Retraction in
-
Retraction. Particokinetics: computational analysis of the superparamagnetic iron oxide nanoparticles deposition process.Int J Nanomedicine. 2012;7:5107. doi: 10.2147/IJN.S37825. Epub 2012 Sep 21. Int J Nanomedicine. 2012. PMID: 23049257 Free PMC article. No abstract available.
Abstract
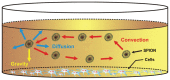
Background: Nanoparticles in suspension are often utilized for intracellular labeling and evaluation of toxicity in experiments conducted in vitro. The purpose of this study was to undertake a computational modeling analysis of the deposition kinetics of a magnetite nanoparticle agglomerate in cell culture medium.
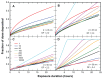
Methods: Finite difference methods and the Crank-Nicolson algorithm were used to solve the equation of mass transport in order to analyze concentration profiles and dose deposition. Theoretical data were confirmed by experimental magnetic resonance imaging.
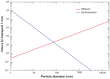
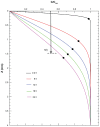
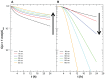
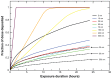
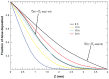
Results: Different behavior in the dose fraction deposited was found for magnetic nanoparticles up to 50 nm in diameter when compared with magnetic nanoparticles of a larger diameter. Small changes in the dispersion factor cause variations of up to 22% in the dose deposited. The experimental data confirmed the theoretical results.
Conclusion: These findings are important in planning for nanomaterial absorption, because they provide valuable information for efficient intracellular labeling and control toxicity. This model enables determination of the in vitro transport behavior of specific magnetic nanoparticles, which is also relevant to other models that use cellular components and particle absorption processes.
Keywords: agglomerates; cellular labeling; computational modeling; diffusion; magnetic resonance imaging; magnetite; nanoparticles; sedimentation.
Figures



References
-
- Sousa MH, Rubim JC, Sobrinho PG, Tourinho FA. Biocompatible magnetic fluid precursors based on aspartic and glutamic acid modified maghemite nanostructures. J Magn Magn Mater. 2001;225:67–72.
-
- Tartaj P, Morales MP, Veintemillas-Verdaguer S, Gonzáles-Carreno T, Serna CJ. The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2003;36:R182–R197.
-
- Gupta AK, Wells S. Surface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studies. IEEE Trans Nanobioscience. 2004;3:66–73. - PubMed
-
- Kopelman R, Koo Y-EL, Philbert M, et al. Multifunctional nanoparticle platforms for in vivo MRI enhancement and photodynamic therapy of a rat brain cancer. J Magn Magn Mater. 2005;293:404–410.
-
- Bonnemain B. Superparamagnetic agents in magnetic resonance imaging: physicochemical characteristics and clinical applications. J Drug Target. 1998;6:167–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

