Omalizumab: clinical use for the management of asthma
- PMID: 22745565
- PMCID: PMC3382304
- DOI: 10.4137/CCRPM.S7793
Omalizumab: clinical use for the management of asthma
Abstract
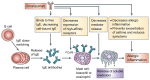
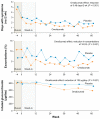
Omalizumab, a humanized monoclonal antibody that binds circulating IgE antibody, is a treatment option for patients with moderate to severe allergic asthma whose asthma is poorly controlled with inhaled corticosteroids and inhaled long-acting β(2) agonist bronchodilators. This review considers the mechanism of action, pharmacokinetics, efficacy, safety and place in management of omalizumab in asthma and focuses particularly on key articles published over the last three years. Omalizumab reduces IgE mediated airway inflammation and its effect on airway remodeling is under investigation. Recent long-term clinical trials confirm the benefits of omalizumab in reducing exacerbations and symptoms in adults and in children with moderate to severe allergic asthma. No clinical or immunological factor consistently predicts a good therapeutic response to omalizumab in allergic asthma. In responders, the duration of treatment is unclear. The main adverse effect of omalizumab is anaphylaxis, although this occurs infrequently. Preliminary data from a five-year safety study has raised concerns about increased cardiovascular events and a final report is awaited. Clinical trials are in progress to determine whether omalizumab has efficacy in the treatment of non-allergic asthma.
Keywords: IgE; allergic asthma; omalizumab; severe asthma.
Figures
References
-
- GINA report global strategy for asthma management prevention. 2010. [Accessed February 25, 2012]. http://www.ginasthma.com.
-
- British guideline on the management of asthma. British Thoracic Society Scottish Intercollegiate Guidelines Network. Thorax. 2008;63:iv, 1–121. - PubMed
-
- Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin. Immunol. 2007;120(5 Suppl 1):S94–138. - PubMed
-
- Dockrell M, Partridge M, Valovirta E. The limitations of severe asthma: the results of a European survey. Allergy. 2007;62(2):134–41. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

