Oridonin up-regulates expression of P21 and induces autophagy and apoptosis in human prostate cancer cells
- PMID: 22745580
- PMCID: PMC3385012
- DOI: 10.7150/ijbs.4554
Oridonin up-regulates expression of P21 and induces autophagy and apoptosis in human prostate cancer cells
Abstract
Background: Oridonin (ORI) could inhibit the proliferation and induce apoptosis in various cancer cell lines. However, the mechanism is not fully understood.
Methods: Human prostate cancer (HPC) cells were cultured in vitro and cell viability was detected by the CCK-8 assay. The ultrastructure changes were observed under transmission electron microscope (TEM). Chemical staining with acridine orange (AO), MDC or DAPI was used to detect acidic vesicular organelles (AVOs) and alternation of DNA. Expression of LC3 and P21 was detected by Western Blot. Apoptotic rates and cell cycle arrest were detected by FACS.
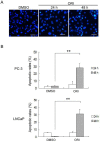
Results: Our study demonstrated that after ORI treatment, the proliferations of human prostate cancer (HPC) cell lines PC-3 and LNCaP were inhibited in a concentration and time-dependent manner. ORI induced cell cycle arrest at the G2/M phase. A large number of autophagosomes with double-membrane structure and acidic vesicular organelles (AVOs) were detected in the cytoplasm of HPC cells treated with ORI for 24 hours. ORI resulted in the conversion of LC3-I to LC3-II and recruitment of LC3-II to the autophagosomal membranes. Autophagy inhibitor 3-methyladenine (3-MA) reduced AVOs formation and inhibited LC3-I to LC3-II conversion. At 48 h, DNA fragmentation, chromatin condensation and disappearance of surface microvilli were detected in ORI-treated cells. ORI induced a significant increase in the number of apoptotic cells (PC-3: 5.4% to 27.0%, LNCaP: 5.3% to 31.0%). Promoting autophagy by nutrient starvation increased cell viability, while inhibition of autophagy by 3-MA promoted cell death. The expression of P21 was increased by ORI, which could be completely reversed by the inhibition of autophagy.
Conclusions: Our findings indicated that autophagy occurred before the onset of apoptosis and protected cancer cells in ORI-treated HPC cells. P21 was involved in ORI-induced autophagy and apoptosis. Our results provide an experimental basis for understand the anti-tumor mechanism of ORI as treatment for prostate cancer.
Keywords: P21.; apoptosis; autophagy; oridonin; prostate cancer.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62:3–12. - PubMed
-
- Marks LS, DiPaola RS, Nelson P. et al. PC-SPES: herbal formulation for prostate cancer. Urology. 2002;60:369–75. - PubMed
-
- Cui Q, Tashiro S, Onodera S. et al. Augmentation of oridonin-induced apoptosis observed with reduced autophagy. J Pharmacol Sci. 2006;101:230–9. - PubMed
-
- Cui Q, Tashiro S, Onodera S. et al. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biol Pharm Bull. 2007;30:859–64. - PubMed
-
- Li D, Cui Q, Chen SG. et al. Inactivation of ras and changes of mitochondrial membrane potential contribute to oridonin-induced autophagy in a431 cells. J Pharmacol Sci. 2007;105:22–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

