Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition
- PMID: 22745588
- PMCID: PMC3384429
- DOI: 10.1596/neo.12432
Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition
Abstract
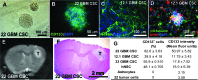
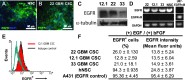
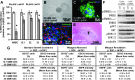
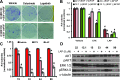
Epidermal growth factor receptor (EGFR) signaling is strongly implicated in glioblastoma (GBM) tumorigenesis. However, molecular agents targeting EGFR have demonstrated minimal efficacy in clinical trials, suggesting the existence of GBM resistance mechanisms. GBM cells with stem-like properties (CSCs) are highly efficient at tumor initiation and exhibit therapeutic resistance. In this study, GBMCSC lines showed sphere-forming and tumor initiation capacity after EGF withdrawal from cell culture media, compared with normal neural stem cells that rapidly perished after EGF withdrawal. Compensatory activation of related ERBB family receptors (ERBB2 and ERBB3) was observed in GBM CSCs deprived of EGFR signal (EGF deprivation or cetuximab inhibition), suggesting an intrinsic GBM resistance mechanism for EGFR-targeted therapy. Dual inhibition of EGFR and ERBB2 with lapatinib significantly reduced GBM proliferation in colony formation assays compared to cetuximab-mediated EGFR-specific inhibition. Phosphorylation of downstream ERBB signaling components (AKT, ERK1/2) and GBM CSC proliferation were inhibited by lapatinib. Collectively, these findings show that GBM therapeutic resistance to EGFR inhibitors may be explained by compensatory activation of EGFR-related family members (ERBB2, ERBB3) enabling GBM CSC proliferation, and therefore simultaneous blockade of multiple ERBB family members may be required for more efficacious GBM therapy.
Figures
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. - PubMed
-
- Lassman AB. Molecular biology of gliomas. Curr Neurol Neurosci Rep. 2004;4:228–233. - PubMed
-
- Aldape KD, Ballman K, Furth A, Buckner JC, Giannini C, Burger PC, Scheithauer BW, Jenkins RB, James CD. Immunohistochemical detection of EGFRvIII in high malignancy grade astrocytomas and evaluation of prognostic significance. J Neuropathol Exp Neurol. 2004;63:700–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
