Characterization of muscle spindle afferents in the adult mouse using an in vitro muscle-nerve preparation
- PMID: 22745708
- PMCID: PMC3380032
- DOI: 10.1371/journal.pone.0039140
Characterization of muscle spindle afferents in the adult mouse using an in vitro muscle-nerve preparation
Abstract
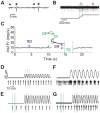
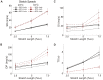
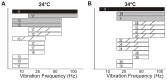
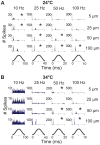
We utilized an in vitro adult mouse extensor digitorum longus (EDL) nerve-attached preparation to characterize the responses of muscle spindle afferents to ramp-and-hold stretch and sinusoidal vibratory stimuli. Responses were measured at both room (24°C) and muscle body temperature (34°C). Muscle spindle afferent static firing frequencies increased linearly in response to increasing stretch lengths to accurately encode the magnitude of muscle stretch (tested at 2.5%, 5% and 7.5% of resting length [Lo]). Peak firing frequency increased with ramp speeds (20% Lo/sec, 40% Lo/sec, and 60% Lo/sec). As a population, muscle spindle afferents could entrain 1:1 to sinusoidal vibrations throughout the frequency (10-100 Hz) and amplitude ranges tested (5-100 µm). Most units preferentially entrained to vibration frequencies close to their baseline steady-state firing frequencies. Cooling the muscle to 24°C decreased baseline firing frequency and units correspondingly entrained to slower frequency vibrations. The ramp component of stretch generated dynamic firing responses. These responses and related measures of dynamic sensitivity were not able to categorize units as primary (group Ia) or secondary (group II) even when tested with more extreme length changes (10% Lo). We conclude that the population of spindle afferents combines to encode stretch in a smoothly graded manner over the physiological range of lengths and speeds tested. Overall, spindle afferent response properties were comparable to those seen in other species, supporting subsequent use of the mouse genetic model system for studies on spindle function and dysfunction in an isolated muscle-nerve preparation.
Conflict of interest statement
Figures

Similar articles
-
Acetylcholine receptors in the equatorial region of intrafusal muscle fibres modulate mouse muscle spindle sensitivity.J Physiol. 2019 Apr;597(7):1993-2006. doi: 10.1113/JP277139. Epub 2019 Feb 13. J Physiol. 2019. PMID: 30673133 Free PMC article.
-
Evidence from the use of vibration during procaine nerve block that the spindle group II fibres contribute excitation to the tonic stretch reflex of the decerebrate cat.J Physiol. 1973 Dec;235(2):371-408. doi: 10.1113/jphysiol.1973.sp010392. J Physiol. 1973. PMID: 4271734 Free PMC article.
-
Regeneration and recovery of cat muscle spindles after devascularization.J Physiol. 1990 May;424:27-39. doi: 10.1113/jphysiol.1990.sp018053. J Physiol. 1990. PMID: 2144024 Free PMC article.
-
Vesicle-released glutamate is necessary to maintain muscle spindle afferent excitability but not dynamic sensitivity in adult mice.J Physiol. 2021 Jun;599(11):2953-2967. doi: 10.1113/JP281182. Epub 2021 Apr 18. J Physiol. 2021. PMID: 33749829 Free PMC article.
-
Mechanosensory encoding in ex vivo muscle-nerve preparations.Exp Physiol. 2024 Jan;109(1):35-44. doi: 10.1113/EP090763. Epub 2023 Apr 29. Exp Physiol. 2024. PMID: 37119460 Free PMC article. Review.
Cited by
-
Diet induced obesity alters muscle spindle afferent function in adult mice.PLoS One. 2018 May 2;13(5):e0196832. doi: 10.1371/journal.pone.0196832. eCollection 2018. PLoS One. 2018. PMID: 29718979 Free PMC article.
-
Methodological advances for studying gamma motor neurons.Curr Opin Physiol. 2021 Feb;19:135-140. doi: 10.1016/j.cophys.2020.10.002. Epub 2020 Oct 14. Curr Opin Physiol. 2021. PMID: 36561377 Free PMC article.
-
Differential encoding of mammalian proprioception by voltage-gated sodium channels.Sci Adv. 2025 Jan 10;11(2):eads6660. doi: 10.1126/sciadv.ads6660. Epub 2025 Jan 8. Sci Adv. 2025. PMID: 39772670 Free PMC article.
-
A Conceptual Exploration of Hamstring Muscle-Tendon Functioning during the Late-Swing Phase of Sprinting: The Importance of Evidence-Based Hamstring Training Frameworks.Sports Med. 2023 Dec;53(12):2321-2346. doi: 10.1007/s40279-023-01904-2. Epub 2023 Sep 5. Sports Med. 2023. PMID: 37668895 Free PMC article. Review.
-
Piezo2 is the principal mechanotransduction channel for proprioception.Nat Neurosci. 2015 Dec;18(12):1756-62. doi: 10.1038/nn.4162. Epub 2015 Nov 9. Nat Neurosci. 2015. PMID: 26551544 Free PMC article.
References
-
- Miwa T, Miwa Y, Kanda K. Dynamic and static sensitivities of muscle spindle primary endings in aged rats to ramp stretch. Neuroscience Letters. 1995;201:179–182. - PubMed
-
- Winarakwong L, Muramoto T, Soma K, Takano Y. Age-related changes and the possible adaptability of rat jaw muscle spindles: immunohistochemical and fine structural studies. Archives of Histology and Cytology. 2004;67:227–240. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

