Stimulation of the sphenopalatine ganglion induces reperfusion and blood-brain barrier protection in the photothrombotic stroke model
- PMID: 22745798
- PMCID: PMC3382129
- DOI: 10.1371/journal.pone.0039636
Stimulation of the sphenopalatine ganglion induces reperfusion and blood-brain barrier protection in the photothrombotic stroke model
Abstract
Purpose: The treatment of stroke remains a challenge. Animal studies showing that electrical stimulation of the sphenopalatine ganglion (SPG) exerts beneficial effects in the treatment of stroke have led to the initiation of clinical studies. However, the detailed effects of SPG stimulation on the injured brain are not known.
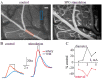
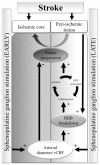
Methods: The effect of acute SPG stimulation was studied by direct vascular imaging, fluorescent angiography and laser Doppler flowmetry in the sensory motor cortex of the anaesthetized rat. Focal cerebral ischemia was induced by the rose bengal (RB) photothrombosis method. In chronic experiments, SPG stimulation, starting 15 min or 24 h after photothrombosis, was given for 3 h per day on four consecutive days. Structural damage was assessed using histological and immunohistochemical methods. Cortical functions were assessed by quantitative analysis of epidural electro-corticographic (ECoG) activity continuously recorded in behaving animals.
Results: Stimulation induced intensity- and duration-dependent vasodilation and increased cerebral blood flow in both healthy and photothrombotic brains. In SPG-stimulated rats both blood brain-barrier (BBB) opening, pathological brain activity and lesion volume were attenuated compared to untreated stroke animals, with no apparent difference in the glial response surrounding the necrotic lesion.
Conclusion: SPG-stimulation in rats induces vasodilation of cortical arterioles, partial reperfusion of the ischemic lesion, and normalization of brain functions with reduced BBB dysfunction and stroke volume. These findings support the potential therapeutic effect of SPG stimulation in focal cerebral ischemia even when applied 24 h after stroke onset and thus may extend the therapeutic window of currently administered stroke medications.
Conflict of interest statement
Figures




References
-
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends in Neurosciences 22: 391–397. Available. 1999. http://www.ncbi.nlm.nih.gov/pubmed/10441299. - PubMed
-
- Kastrup A, Engelhorn T, Beaulieu C, de Crespigny A, Moseley ME. Dynamics of cerebral injury, perfusion, and blood-brain barrier changes after temporary and permanent middle cerebral artery occlusion in the rat. Journal of the neurological sciences 166: 91–99. 1999. Available: http://www.jns-journal.com/article/S0022-510X(99)00121-5/abstract. Accessed 21 February 2012. - PubMed
-
- Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nature reviews Neurology 6: 393–403. Available. 2010. http://www.ncbi.nlm.nih.gov/pubmed/20551947. - PMC - PubMed
-
- Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 36: 1432–1438. Available. 2005. http://www.ncbi.nlm.nih.gov/pubmed/15961709. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

