Neutrophil paralysis in Plasmodium vivax malaria
- PMID: 22745844
- PMCID: PMC3383745
- DOI: 10.1371/journal.pntd.0001710
Neutrophil paralysis in Plasmodium vivax malaria
Abstract
Background: The activation of innate immune responses by Plasmodium vivax results in activation of effector cells and an excessive production of pro-inflammatory cytokines that may culminate in deleterious effects. Here, we examined the activation and function of neutrophils during acute episodes of malaria.
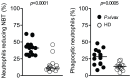
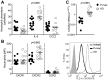
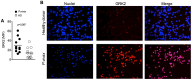
Materials and methods: Blood samples were collected from P. vivax-infected patients at admission (day 0) and 30-45 days after treatment with chloroquine and primaquine. Expression of activation markers and cytokine levels produced by highly purified monocytes and neutrophils were measured by the Cytometric Bead Assay. Phagocytic activity, superoxide production, chemotaxis and the presence of G protein-coupled receptor (GRK2) were also evaluated in neutrophils from malaria patients.
Principal findings: Both monocytes and neutrophils from P. vivax-infected patients were highly activated. While monocytes were found to be the main source of cytokines in response to TLR ligands, neutrophils showed enhanced phagocytic activity and superoxide production. Interestingly, neutrophils from the malaria patients expressed high levels of GRK2, low levels of CXCR2, and displayed impaired chemotaxis towards IL-8 (CXCL8).
Conclusion: Activated neutrophils from malaria patients are a poor source of pro-inflammatory cytokines and display reduced chemotactic activity, suggesting a possible mechanism for an enhanced susceptibility to secondary bacterial infection during malaria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. - PubMed
-
- WHO. 2010. World Malaria Report.
-
- Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol. 2006;6:895–906. - PubMed
-
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. - PubMed
-
- Kwiatkowski D, Hill AV, Sambou I, Twumasi P, Castracane J, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

