Nitrosothiols in the immune system: signaling and protection
- PMID: 22746191
- PMCID: PMC3518543
- DOI: 10.1089/ars.2012.4765
Nitrosothiols in the immune system: signaling and protection
Abstract
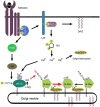
Significance: In the immune system, nitric oxide (NO) has been mainly associated with antibacterial defenses exerted through oxidative, nitrosative, and nitrative stress and signal transduction through cyclic GMP-dependent mechanisms. However, S-nitrosylation is emerging as a post-translational modification (PTM) involved in NO-mediated cell signaling.
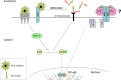
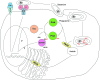
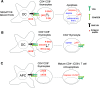
Recent advances: Precise roles for S-nitrosylation in signaling pathways have been described both for innate and adaptive immunity. Denitrosylation may protect macrophages from their own S-nitrosylation, while maintaining nitrosative stress compartmentalized in the phagosomes. Nitrosothiols have also been shown to be beneficial in experimental models of autoimmune diseases, mainly through their role in modulating T-cell differentiation and function.
Critical issues: Relationship between S-nitrosylation, other thiol redox PTMs, and other NO-signaling pathways has not been always taken into account, particularly in the context of immune responses. Methods for assaying S-nitrosylation in individual proteins and proteomic approaches to study the S-nitrosoproteome are constantly being improved, which helps to move this field forward.
Future directions: Integrated studies of signaling pathways in the immune system should consider whether S-nitrosylation/denitrosylation processes are among the PTMs influencing the activity of key signaling and adaptor proteins. Studies in pathophysiological scenarios will also be of interest to put these mechanisms into broader contexts. Interventions modulating nitrosothiol levels in autoimmune disease could be investigated with a view to developing new therapies.
Figures


Similar articles
-
Proteomic approaches to evaluate protein S-nitrosylation in disease.Mass Spectrom Rev. 2014 Jan-Feb;33(1):7-20. doi: 10.1002/mas.21373. Epub 2013 Jun 15. Mass Spectrom Rev. 2014. PMID: 23775552 Review.
-
The role of thioredoxin in the regulation of cellular processes by S-nitrosylation.Biochim Biophys Acta. 2012 Jun;1820(6):689-700. doi: 10.1016/j.bbagen.2011.08.012. Epub 2011 Aug 22. Biochim Biophys Acta. 2012. PMID: 21878369 Review.
-
S-nitrosothiols and the S-nitrosoproteome of the cardiovascular system.Antioxid Redox Signal. 2013 Jan 20;18(3):270-87. doi: 10.1089/ars.2012.4744. Epub 2012 Sep 5. Antioxid Redox Signal. 2013. PMID: 22770551 Free PMC article. Review.
-
Protein denitrosylation: enzymatic mechanisms and cellular functions.Nat Rev Mol Cell Biol. 2009 Oct;10(10):721-32. doi: 10.1038/nrm2764. Epub 2009 Sep 9. Nat Rev Mol Cell Biol. 2009. PMID: 19738628 Review.
-
Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling.Antioxid Redox Signal. 2019 Apr 1;30(10):1331-1351. doi: 10.1089/ars.2017.7403. Epub 2018 Jan 10. Antioxid Redox Signal. 2019. PMID: 29130312 Free PMC article. Review.
Cited by
-
S-nitrosylation: specificity, occupancy, and interaction with other post-translational modifications.Antioxid Redox Signal. 2013 Oct 10;19(11):1209-19. doi: 10.1089/ars.2012.5056. Epub 2013 Jan 4. Antioxid Redox Signal. 2013. PMID: 23157187 Free PMC article. Review.
-
S-Nitrosoglutathione Reductase Deficiency Confers Improved Survival and Neurological Outcome in Experimental Cerebral Malaria.Infect Immun. 2017 Aug 18;85(9):e00371-17. doi: 10.1128/IAI.00371-17. Print 2017 Sep. Infect Immun. 2017. PMID: 28674030 Free PMC article.
-
Regulation of Ras Signaling by S-Nitrosylation.Antioxidants (Basel). 2023 Aug 4;12(8):1562. doi: 10.3390/antiox12081562. Antioxidants (Basel). 2023. PMID: 37627556 Free PMC article. Review.
-
Transflammation: Innate immune signaling in nuclear reprogramming.Adv Drug Deliv Rev. 2017 Oct 1;120:133-141. doi: 10.1016/j.addr.2017.09.010. Epub 2017 Sep 13. Adv Drug Deliv Rev. 2017. PMID: 28916494 Free PMC article. Review.
-
Female resistance to pneumonia identifies lung macrophage nitric oxide synthase-3 as a therapeutic target.Elife. 2014 Oct 15;3:e03711. doi: 10.7554/eLife.03711. Elife. 2014. PMID: 25317947 Free PMC article.
References
-
- Aiello S. Cassis P. Cassis L. Tomasoni S. Benigni A. Pezzotta A. Cavinato RA. Cugini D. Azzollini N. Mister M. Longaretti L. Thomson AW. Remuzzi G. Noris M. DnIKK2-transfected dendritic cells induce a novel population of inducible nitric oxide synthase-expressing CD4+CD25- cells with tolerogenic properties. Transplantation. 2007;83:474–484. - PubMed
-
- Aiello S. Noris M. Piccinini G. Tomasoni S. Casiraghi F. Bonazzola S. Mister M. Sayegh MH. Remuzzi G. Thymic dendritic cells express inducible nitric oxide synthase and generate nitric oxide in response to self- and alloantigens. J Immunol. 2000;164:4649–4658. - PubMed
-
- Akhand AA. Pu M. Senga T. Kato M. Suzuki H. Miyata T. Hamaguchi M. Nakashima I. Nitric oxide controls src kinase activity through a sulfhydryl group modification-mediated Tyr-527-independent and Tyr-416-linked mechanism. J Biol Chem. 1999;274:25821–25826. - PubMed
-
- Arnelle DR. Stamler JS. NO+, NO, and NO- donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch Biochem Biophys. 1995;318:279–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

