Structure and regulation of the type VI secretion system
- PMID: 22746332
- PMCID: PMC3595004
- DOI: 10.1146/annurev-micro-121809-151619
Structure and regulation of the type VI secretion system
Abstract
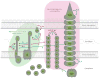
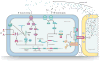
The type VI secretion system (T6SS) is a complex and widespread gram-negative bacterial export pathway with the capacity to translocate protein effectors into a diversity of target cell types. Current structural models of the T6SS indicate that the apparatus is composed of at least two complexes, a dynamic bacteriophage-like structure and a cell-envelope-spanning membrane-associated assembly. How these complexes interact to promote effector secretion and cell targeting remains a major question in the field. As a contact-dependent pathway with specific cellular targets, the T6SS is subject to tight regulation. Thus, the identification of regulatory elements that control T6S expression continues to shape our understanding of the environmental circumstances relevant to its function. This review discusses recent progress toward characterizing T6S structure and regulation.
Figures

References
-
- Abuladze NK, Gingery M, Tsai J, Eiserling FA. Tail length determination in bacteriophage T4. Virology. 1994;199:301–10. - PubMed
-
- Aschtgen MS, Gavioli M, Dessen A, Lloubes R, Cascales E. The SciZ protein anchors the enteroaggregative Escherichia coli type VI secretion system to the cell wall. Mol Microbiol. 2010;75:886–99. Provides evidence that four T6S proteins assemble a membrane-associated complex. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

