Expression of cyclooxygenase-1 (COX-1) and COX-2 in human male gametes from normal patients, and those with varicocele and diabetes: a potential molecular marker for diagnosing male infertility disorders
- PMID: 22747653
- PMCID: PMC3458626
- DOI: 10.1111/j.1469-7580.2012.01534.x
Expression of cyclooxygenase-1 (COX-1) and COX-2 in human male gametes from normal patients, and those with varicocele and diabetes: a potential molecular marker for diagnosing male infertility disorders
Abstract
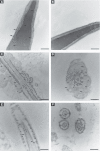
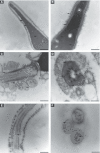
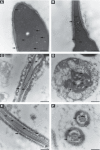
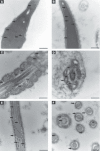
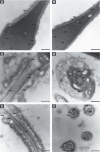
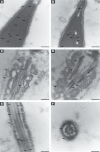
Rising rates of varicocele and diabetes mellitus (DM) pose a significant problem to human fertility. Recent studies have pointed out the impact of cyclooxygenase (COX) in the regulation of testicular function and male fertility. Prominent COX-2 expression has been described recently in the testes of infertile patients, but little is known about the role and identity of COX isoforms in human sperm under certain disease states such as varicocele and DM. We therefore examined the expression profile and ultrastructural localization of COX-1 and COX-2 concomitantly in semen samples from healthy donors, and patients with varicocele and DM. Using Western blotting assay, 'varicocele' and 'diabetic' sperm showed enhanced COX isoforms expression with respect to the 'healthy' sperm. Immunogold labeling revealed human sperm anatomical regions containing COX-1 and COX-2, confirming their increased expression in pathological samples. Our data demonstrate that both COX isoforms are upregulated in the spermatozoa of varicocele and diabetic patients, suggesting the harmful effect of the diseases also at the sperm molecular level, going beyond the abnormal morphology described to date. In conclusion, COX enzymes may possess a biological relevance in the pathogenesis and/or maintenance of male factor infertility associated with varicocele and DM, and may be considered additional molecular markers for the diagnosis of male infertility disorders.
© 2012 The Authors. Journal of Anatomy © 2012 Anatomical Society.
Figures

References
-
- Adegboyega PA, Ololade O. Immunohistochemical expression of cyclooxygenase-2 in normal kidneys. Appl Immunohistochem Mol Morphol. 2004;12:71–74. - PubMed
-
- Agbaje IM, Rogers DA, McVicar CM, et al. Insulin dependent diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007;22:1871–1877. - PubMed
-
- Aitken RJ, Kelly RW. Analysis of the direct effects of prostaglandins on human sperm function. J Reprod Fertil. 1985;73:139–146. - PubMed
-
- Aitken RJ, Irvine S, Kelly RW. Significance of intracellular calcium and cyclic adenosine 30,50-monophosphate in the mechanisms by which prostaglandins influence human sperm function. J Reprod Fertil. 1986;77:451–462. - PubMed
-
- Aquila S, Sisci D, Gentile ME, et al. Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab. 2002;87:3385–3390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

