[11C]phenytoin revisited: synthesis by [11C]CO carbonylation and first evaluation as a P-gp tracer in rats
- PMID: 22747744
- PMCID: PMC3506555
- DOI: 10.1186/2191-219X-2-36
[11C]phenytoin revisited: synthesis by [11C]CO carbonylation and first evaluation as a P-gp tracer in rats
Abstract
Background: At present, several positron emission tomography (PET) tracers are in use for imaging P-glycoprotein (P-gp) function in man. At baseline, substrate tracers such as R-[11C]verapamil display low brain concentrations with a distribution volume of around 1. [11C]phenytoin is supposed to be a weaker P-gp substrate, which may lead to higher brain concentrations at baseline. This could facilitate assessment of P-gp function when P-gp is upregulated. The purpose of this study was to synthesize [11C]phenytoin and to characterize its properties as a P-gp tracer.
Methods: [11C]CO was used to synthesize [11C]phenytoin by rhodium-mediated carbonylation. Metabolism and, using PET, brain pharmacokinetics of [11C]phenytoin were studied in rats. Effects of P-gp function on [11C]phenytoin uptake were assessed using predosing with tariquidar.
Results: [11C]phenytoin was synthesized via [11C]CO in an overall decay-corrected yield of 22 ± 4%. At 45 min after administration, 19% and 83% of radioactivity represented intact [11C]phenytoin in the plasma and brain, respectively. Compared with baseline, tariquidar predosing resulted in a 45% increase in the cerebral distribution volume of [11C]phenytoin.
Conclusions: Using [11C]CO, the radiosynthesis of [11C]phenytoin could be improved. [11C]phenytoin appeared to be a rather weak P-gp substrate.
Figures
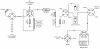








References
LinkOut - more resources
Full Text Sources
Miscellaneous

