Functional monoclonal antibody acts as a biased agonist by inducing internalization of metabotropic glutamate receptor 7
- PMID: 22747985
- PMCID: PMC3514759
- DOI: 10.1111/j.1476-5381.2012.02090.x
Functional monoclonal antibody acts as a biased agonist by inducing internalization of metabotropic glutamate receptor 7
Abstract
Background and purpose: The mGlu(7) receptors are strategically located at the site of vesicle fusion where they modulate the release of the main excitatory and inhibitory neurotransmitters. Consequently, they are implicated in the underlying pathophysiology of CNS diseases such as epilepsy and stress-related psychiatric disorders. Here, we characterized a selective, potent and functional anti-mGlu(7) monoclonal antibody, MAB1/28, that triggers receptor internalization.
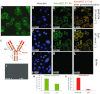
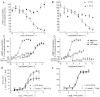
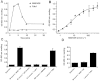
Experimental approach: MAB1/28's activity was investigated using Western blot and direct immunofluorescence on live cells, in vitro pharmacology by functional cAMP and [(35) S]-GTPγ binding assays, the kinetics of IgG-induced internalization by image analysis, and the activation of the ERK1/2 by elisa.
Key results: mGlu(7) /mGlu(6) chimeric studies located the MAB1/28 binding site at the extracellular amino-terminus of mGlu(7) . MAB1/28 potently antagonized both orthosteric and allosteric agonist-induced inhibition of cAMP accumulation. The potency of the antagonistic actions was similar to the potency in triggering receptor internalization. The internalization mechanism occurred via a pertussis toxin-insensitive pathway and did not require Gα(i) protein activation. MAB1/28 activated ERK1/2 with potency similar to that for receptor internalization. The requirement of a bivalent receptor binding mode for receptor internalizations suggests that MAB1/28 modulates mGlu(7) dimers.
Conclusions and implications: We obtained evidence for an allosteric-biased agonist activity triggered by MAB1/28, which activates a novel IgG-mediated GPCR internalization pathway that is not utilized by small molecule, orthosteric or allosteric agonists. Thus, MAB1/28 provides an invaluable biological tool for probing mGlu(7) function and selective activation of its intracellular trafficking.
© 2012 F. Hoffmann-La Roche AG, Basel, Switzerland. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures






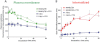
References
-
- Acher FC, Selvam C, Pin JP, Goudet C, Bertrand HO. A critical pocket close to the glutamate binding site of mGlu receptors opens new possibilities for agonist design. Neuropharmacology. 2011;60:102–107. - PubMed
-
- Airas JM, Betz H, El Far O. PKC phosphorylation of a conserved serine residue in the C-terminus of group III metabotropic glutamate receptors inhibits calmodulin binding. FEBS Lett. 2001;494:60–63. - PubMed
-
- Aplin M, Christensen GL, Schneider M, Heydorn A, Gammeltoft S, Kjolbye AL, et al. Differential extracellular signal-regulated kinases 1 and 2 activation by the angiotensin type 1 receptor supports distinct phenotypes of cardiac myocytes. Basic Clin Pharmacol Toxicol. 2007;100:296–301. - PubMed
-
- Bennett TA, Maestas DC, Prossnitz ER. Arrestin binding to the G protein-coupled N-formyl peptide receptor is regulated by the conserved ‘DRY’ sequence. J Biol Chem. 2000;275:24590–24594. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

