XELIRI-bevacizumab versus FOLFIRI-bevacizumab as first-line treatment in patients with metastatic colorectal cancer: a Hellenic Cooperative Oncology Group phase III trial with collateral biomarker analysis
- PMID: 22748098
- PMCID: PMC3466131
- DOI: 10.1186/1471-2407-12-271
XELIRI-bevacizumab versus FOLFIRI-bevacizumab as first-line treatment in patients with metastatic colorectal cancer: a Hellenic Cooperative Oncology Group phase III trial with collateral biomarker analysis
Abstract
Background: The aim was to compare two standard chemotherapy regimens combined with bevacizumab as first-line treatment in patients with metastatic colorectal cancer.
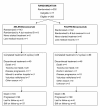
Methods: Patients previously untreated for metastatic disease were randomized in: group A (irinotecan, capecitabine, bevacizumab, every 3 weeks; XELIRI-bevacizumab) and group B (irinotecan, leucovorin, fluorouracil, bevacizumab, every 2 weeks; FOLFIRI-bevacizumab). Primary endpoint was progression-free survival (PFS). Plasma concentrations of nitric oxide, osteopontin, TGF-β1 and VEGF-A were measured at baseline and during treatment.
Results: Among 285 eligible patients, 143 were randomized to group A and 142 to group B. Fifty-five patients (38.5%) in group A and 57 (40.1%) in group B responded (p = 0.81). After a median follow-up of 42 months, median PFS was 10.2 and 10.8 months (p = 0.74), while median OS was 20.0 and 25.3 months (p = 0.099), for groups A and B, respectively. Most frequent grade 3-4 toxicities (group A vs group B) were neutropenia (13% vs 22%, p = 0.053) and diarrhea (19% vs 11%, p = 0.082). Baseline plasma osteopontin concentrations demonstrated prognostic significance for both PFS and OS.
Conclusions: This trial did not show significant differences in efficacy between the groups. However, the toxicity profile was different. Baseline plasma osteopontin concentrations demonstrated independent prognostic significance. (
Registration number: ACTRN12610000270011).
Figures


References
-
- Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. - DOI - PubMed
-
- Köhne CH, De Greve J, Hartmann JT Lang I, Vergauwe P, Becker K, Braumann D, Joosens E, Müller L, Janssens J, Bokemeyer C, Reimer P, Link H, Späth-Schwalbe E, Wilke HJ, Bleiberg H, Van Den Brande J, Debois M, Bethe U, Van Cutsem E. Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Ann Oncol. 2008;19:920–926. doi: 10.1093/annonc/mdm544. - DOI - PubMed
-
- García-Alfonso P, Muñoz-Martin AJ, Alvarez-Suarez S, Jerez-Gilarranz Y, Riesco-Martinez M, Khosravi P, Martin M. Bevacizumab in combination with biweekly capecitabine and irinotecan, as first-line treatment for patients with metastatic colorectal cancer. Br J Cancer. 2010;103:1524–1528. doi: 10.1038/sj.bjc.6605907. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

