Pharmacokinetic analysis of ziconotide (SNX-111), an intrathecal N-type calcium channel blocking analgesic, delivered by bolus and infusion in the dog
- PMID: 22748108
- PMCID: PMC3514653
- DOI: 10.1111/j.1525-1403.2012.00479.x
Pharmacokinetic analysis of ziconotide (SNX-111), an intrathecal N-type calcium channel blocking analgesic, delivered by bolus and infusion in the dog
Abstract
Background and purpose: Ziconotide is a peptide that blocks N-type calcium channels and is antihyperalgesic after intrathecal (IT) delivery. We here characterize the spinal kinetics of IT bolus and infused ziconotide in dog.
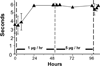
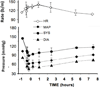
Experimental approach: Male beagle dogs (N= 5) were prepared with chronic IT lumbar injection and cerebrospinal fluid (LCSF) sampling catheters connected to vest-mounted pumps. Each dog received the following: 1) IT bolus ziconotide (10 µg + 1 µCi (3) H-inulin); 2) IT infusion for 48 hours of ziconotide (1 µg/100 µL/hour); 3) IT infusion for 48 hours of ziconotide (5 µg/100 µL/hour); and 4) intravenous injection of ziconotide (0.1 mg/kg). After IT bolus, LCSF ziconotide and inulin showed an initial peak and biphasic (distribution/elimination) clearance (ziconotide T(1/2-α/β) = 0.14 and 1.77 hours, and inulin T(1/2-α/β) = 0.16 and 3.88 hours, respectively). The LCSF : plasma ziconotide concentration ratio was 20,000:1 at 30 min and 30:1 at eight hours. IT infusion of 1 and then 5 µg/hour resulted in LCSF concentrations that peaked by eight hours and remained stable at 343 and 1380 ng/mL, respectively, to the end of the 48-hour infusions. Terminal elimination T(1/2) after termination of continuous infusion was 2.47 hours. Ziconotide LCSF : cisternal CSF : plasma concentration ratios after infusion of 1 and 5 µg/hour were 1:0.017:0.001 and 1:0.015:0.003, respectively. IT infusion of ziconotide at 1 µg/hour inhibited thermal skin twitch by 24 hours and produced modest trembling, ataxia, and decreased arousal. Effects continued through the 48-hour infusion period, increased in magnitude during the subsequent 5 µg/hour infusion periods, and disappeared after drug clearance.
Conclusions and implications: After IT bolus or infusion, ziconotide displays linear kinetics that are consistent with a hydrophilic molecule of approximately 2500 Da that is cleared slightly more rapidly than inulin from the LCSF. Behavioral effects were dose dependent and reversible.
© 2012 International Neuromodulation Society.
Conflict of interest statement
The other authors reported no conflicts of interest.
Figures





Similar articles
-
Ziconotide, an intrathecally administered N-type calcium channel antagonist for the treatment of chronic pain.Pharmacotherapy. 2005 Aug;25(8):1084-94. doi: 10.1592/phco.2005.25.8.1084. Pharmacotherapy. 2005. PMID: 16207099 Review.
-
Pharmacokinetics and pharmacodynamics of intrathecal ziconotide in chronic pain patients.J Clin Pharmacol. 2003 Jun;43(6):624-36. J Clin Pharmacol. 2003. PMID: 12817525 Clinical Trial.
-
Ziconotide, a new N-type calcium channel blocker, administered intrathecally for acute postoperative pain.Reg Anesth Pain Med. 2000 May-Jun;25(3):274-8. doi: 10.1016/s1098-7339(00)90010-5. Reg Anesth Pain Med. 2000. PMID: 10834782 Clinical Trial.
-
Interactions of intrathecally administered ziconotide, a selective blocker of neuronal N-type voltage-sensitive calcium channels, with morphine on nociception in rats.Pain. 2000 Feb;84(2-3):271-81. doi: 10.1016/s0304-3959(99)00214-6. Pain. 2000. PMID: 10666532
-
Ziconotide--a novel neuron-specific calcium channel blocker for the intrathecal treatment of severe chronic pain--a short review.Int J Clin Pharmacol Ther. 2006 Oct;44(10):478-83. doi: 10.5414/cpp44478. Int J Clin Pharmacol Ther. 2006. PMID: 17063978 Review.
Cited by
-
Intrathecal Drug Delivery: Advances and Applications in the Management of Chronic Pain Patient.Front Pain Res (Lausanne). 2022 Jun 16;3:900566. doi: 10.3389/fpain.2022.900566. eCollection 2022. Front Pain Res (Lausanne). 2022. PMID: 35782225 Free PMC article. Review.
-
Effects of arginine 10 to lysine substitution on ω-conotoxin CVIE and CVIF block of Cav2.2 channels.Br J Pharmacol. 2014 Jul;171(13):3313-27. doi: 10.1111/bph.12686. Br J Pharmacol. 2014. PMID: 24628243 Free PMC article.
-
Intrathecal Ziconotide: Dosing and Administration Strategies in Patients With Refractory Chronic Pain.Neuromodulation. 2016 Jul;19(5):522-32. doi: 10.1111/ner.12392. Epub 2016 Feb 9. Neuromodulation. 2016. PMID: 26856969 Free PMC article. Review.
-
The Pharmacology of Spinal Opioids and Ziconotide for the Treatment of Non-Cancer Pain.Curr Neuropharmacol. 2017;15(2):206-216. doi: 10.2174/1570159x14666160210142339. Curr Neuropharmacol. 2017. PMID: 26861471 Free PMC article. Review.
-
A comprehensive review on ziconotide.Heliyon. 2024 May 11;10(10):e31105. doi: 10.1016/j.heliyon.2024.e31105. eCollection 2024 May 30. Heliyon. 2024. PMID: 38779019 Free PMC article. Review.
References
-
- Olivera BM, Gray WR, Zeikus R, McIntosh JM, Varga J, Rivier J, de Santos V, Cruz LJ. Peptide neurotoxins from fish-hunting cone snails. Science. 1985;230:1338–1343. - PubMed
-
- Nadasdi L, Yamashiro D, Chung D, Tarczy-Hornoch K, Adriaenssens P, Ramachandran J. Structure-activity analysis of a Conus peptide blocker of N-type neuronal calcium channels. Biochemistry. 1995;34:8076–8081. - PubMed
-
- Cruz LJ, Olivera BM. Calcium channel antagonists. Omega-conotoxin defines a new high affinity site. J. Biol. Chem. 1986;261:6230–6233. - PubMed
-
- Miljanich GP. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004;11:3029–3040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

