Caveolin-1 suppresses human immunodeficiency virus-1 replication by inhibiting acetylation of NF-κB
- PMID: 22748181
- PMCID: PMC3767293
- DOI: 10.1016/j.virol.2012.05.016
Caveolin-1 suppresses human immunodeficiency virus-1 replication by inhibiting acetylation of NF-κB
Abstract
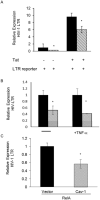
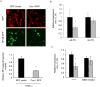
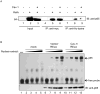
Caveolin-1 is an integral membrane protein primarily responsible for the formation of membrane structures known as caveolae. Caveolae are specialized lipid rafts involved in protein trafficking, cholesterol homeostasis, and a number of signaling functions. It has been demonstrated that caveolin-1 suppresses HIV-1 protein expression. We found that co-transfecting cells with HIV-1 and caveolin-1 constructs, results in a marked decrease in the level of HIV-1 transcription relative to cells transfected with HIV-1 DNA alone. Correspondingly, reduction of endogenous caveolin-1 expression by siRNA-mediated silencing resulted in an enhancement of HIV-1 replication. Further, we observed a loss of caveolin-mediated suppression of HIV-1 transcription in promoter studies with reporters containing mutations in the NF-κB binding site. Our analysis of the posttranslational modification status of the p65 subunit of NF-κB demonstrates hypoacetylation of p65 in the presence of caveolin-1. Since hypoacetylated p65 has been shown to inhibit transcription, we conclude that caveolin-1 inhibits HIV-1 transcription through a NF-κB-dependent mechanism.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Caveolin 1 inhibits HIV replication by transcriptional repression mediated through NF-κB.J Virol. 2011 Jun;85(11):5483-93. doi: 10.1128/JVI.00254-11. Epub 2011 Mar 23. J Virol. 2011. PMID: 21430048 Free PMC article.
-
Allo-antigen stimulated CD8+ T-cells suppress NF-κB and Ets-1 DNA binding activity, and inhibit phosphorylated NF-κB p65 nuclear localization in CD4+ T-cells.Viral Immunol. 2014 Aug;27(6):305-15. doi: 10.1089/vim.2013.0113. Epub 2014 May 20. Viral Immunol. 2014. PMID: 24844121 Free PMC article.
-
NF-κB-Interacting Long Noncoding RNA Regulates HIV-1 Replication and Latency by Repressing NF-κB Signaling.J Virol. 2020 Aug 17;94(17):e01057-20. doi: 10.1128/JVI.01057-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32581100 Free PMC article.
-
The Role of Caveolin 1 in HIV Infection and Pathogenesis.Viruses. 2017 May 26;9(6):129. doi: 10.3390/v9060129. Viruses. 2017. PMID: 28587148 Free PMC article. Review.
-
[Recent progress in the study of HIV-1 transcription factor NF-kappaB and its inhibitors].Yao Xue Xue Bao. 2007 Oct;42(10):1007-12. Yao Xue Xue Bao. 2007. PMID: 18229603 Review. Chinese.
Cited by
-
An association of metabolic syndrome constellation with cellular membrane caveolae.Pathobiol Aging Age Relat Dis. 2014 Feb 12;4. doi: 10.3402/pba.v4.23866. eCollection 2014. Pathobiol Aging Age Relat Dis. 2014. PMID: 24563731 Free PMC article. Review.
-
Identification of molecular sub-networks associated with cell survival in a chronically SIVmac-infected human CD4+ T cell line.Virol J. 2014 Aug 27;11:152. doi: 10.1186/1743-422X-11-152. Virol J. 2014. PMID: 25163480 Free PMC article.
-
The Potential Contribution of Caveolin 1 to HIV Latent Infection.Pathogens. 2020 Oct 27;9(11):896. doi: 10.3390/pathogens9110896. Pathogens. 2020. PMID: 33121153 Free PMC article. Review.
-
Multifaceted Functions of Host Cell Caveolae/Caveolin-1 in Virus Infections.Viruses. 2020 Apr 26;12(5):487. doi: 10.3390/v12050487. Viruses. 2020. PMID: 32357558 Free PMC article. Review.
-
Caveolin-1 in lipid rafts interacts with dengue virus NS3 during polyprotein processing and replication in HMEC-1 cells.PLoS One. 2014 Mar 18;9(3):e90704. doi: 10.1371/journal.pone.0090704. eCollection 2014. PLoS One. 2014. PMID: 24643062 Free PMC article.
References
-
- Arakawa R, Abe-Dohmae S, Asai M, Ito JI, Yokoyama S. Involvement ofcaveolin-1 in cholesterol enrichment of high density lipoprotein during its assembly by apolipoprotein and THP-1 cells. J Lipid Res. 2000;41:1952–1962. - PubMed
-
- Benferhat R, Krust B, Rey-Cuille MA, Hovanessian AG. The caveolin-1 binding domain of HIV-1 glycoprotein gp41 (CBD1) contains several overlapping neutralizing epitopes. Vaccine. 2009a;27:3620–3630. - PubMed
-
- Benferhat R, Martinon F, Krust B, Le Grand R, Hovanessian AG. The CBD1 peptide corresponding to the caveolin-1 binding domain of HIV-1 glycoprotein gp41 elicits neutralizing antibodies in cynomolgus macaques when administered with the tetanus T helper epitope. Mol Immunol. 2009b;46:705–712. - PubMed
-
- Benferhat R, Sanchez-Martinez S, Nieva JL, Briand JP, Hovanessian AG. The immunogenic CBD1 peptide corresponding to the caveolin-1 binding domain in HIV^1envelope gp41 has the capacity to penetrate the cell membrane and bind caveolin-1. Mol Immunol. 2008;45:1963–1975. - PubMed
-
- Bennasser Y, Contreras X, Moreau M, Le Clerc C, Badou A, Bahraoui E. HIV-1 Tat protein induces IL-10 production by human monocytes: implications of the PKC and calcium pathway. J Soc Biol. 2001;195:319–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

