Dapson in heterocyclic chemistry, part VIII: synthesis, molecular docking and anticancer activity of some novel sulfonylbiscompounds carrying biologically active 1,3-dihydropyridine, chromene and chromenopyridine moieties
- PMID: 22748424
- PMCID: PMC3543391
- DOI: 10.1186/1752-153X-6-64
Dapson in heterocyclic chemistry, part VIII: synthesis, molecular docking and anticancer activity of some novel sulfonylbiscompounds carrying biologically active 1,3-dihydropyridine, chromene and chromenopyridine moieties
Abstract
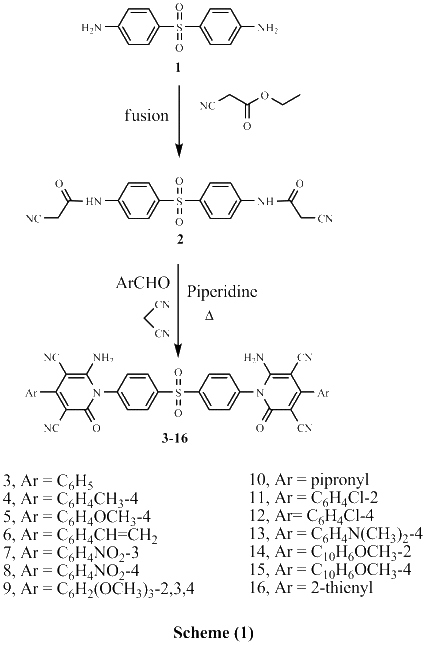
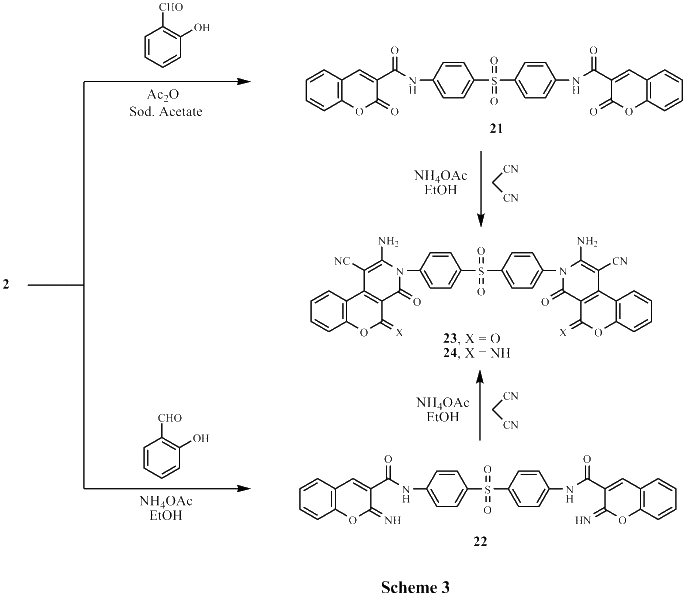
Several new sulfonebiscompounds having a biologically active 1,2-dihydropyridine-2-one 3-19, acrylamide 20, chromene 21, 22 and chromenopyridine 23, 24 moieties were synthesized and evaluated as potential anticancer agents. The structures of the products were confirmed via elemental analyses and spectral data. The screening tests showed that many of the biscompounds obtained exhibited good anticancer activity against human breast cell line (MCF7) comparable to doxorubicin which was used as reference drug. Compounds 11, 17 and 24 showed IC50 values 35.40 μM, 29.86 μM and 30.99 μM, respectively. In order to elucidate the mechanism of action of the synthesized compounds as anticancer agents, docking on the active site of farnesyltransferase and arginine methyltransferase was also performed and good results were obtained.
Figures

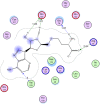
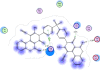
References
-
- Fan N, Evans DB, Rank KB, Thomas RC, Tarpley WG, Sharma SK. Mechanism of resistance to U-90152 S and sensitization to L-697,661 by a proline to leucine change at residue 236 of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. FEBS Lett. 1995;359:233–238. doi: 10.1016/0014-5793(95)00051-A. - DOI - PubMed
-
- Emini EA, Staszewski S, Schneider CL, Waterbury JA, Schleif WA, Goehler R, Deussen A, Duerr S, Massari FE, Calandra GB, Hoffstedt B, Byrnes VW. 94 Combination therapy with AZT prevents selection of HIV-1 variants that are highly resistant to the nonnucleoside reverse transcriptase inhibitor L-697, 661. Antiviral Res. 1993;20:94.
LinkOut - more resources
Full Text Sources

