Bacteriophage protein-protein interactions
- PMID: 22748812
- PMCID: PMC3461333
- DOI: 10.1016/B978-0-12-394438-2.00006-2
Bacteriophage protein-protein interactions
Abstract
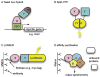
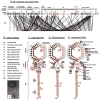
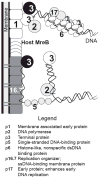
Bacteriophages T7, λ, P22, and P2/P4 (from Escherichia coli), as well as ϕ29 (from Bacillus subtilis), are among the best-studied bacterial viruses. This chapter summarizes published protein interaction data of intraviral protein interactions, as well as known phage-host protein interactions of these phages retrieved from the literature. We also review the published results of comprehensive protein interaction analyses of Pneumococcus phages Dp-1 and Cp-1, as well as coliphages λ and T7. For example, the ≈55 proteins encoded by the T7 genome are connected by ≈43 interactions with another ≈15 between the phage and its host. The chapter compiles published interactions for the well-studied phages λ (33 intra-phage/22 phage-host), P22 (38/9), P2/P4 (14/3), and ϕ29 (20/2). We discuss whether different interaction patterns reflect different phage lifestyles or whether they may be artifacts of sampling. Phages that infect the same host can interact with different host target proteins, as exemplified by E. coli phage λ and T7. Despite decades of intensive investigation, only a fraction of these phage interactomes are known. Technical limitations and a lack of depth in many studies explain the gaps in our knowledge. Strategies to complete current interactome maps are described. Although limited space precludes detailed overviews of phage molecular biology, this compilation will allow future studies to put interaction data into the context of phage biology.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



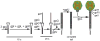



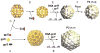

Similar articles
-
The protein interaction network of bacteriophage lambda with its host, Escherichia coli.J Virol. 2013 Dec;87(23):12745-55. doi: 10.1128/JVI.02495-13. Epub 2013 Sep 18. J Virol. 2013. PMID: 24049175 Free PMC article.
-
The interactome of Streptococcus pneumoniae and its bacteriophages show highly specific patterns of interactions among bacteria and their phages.Sci Rep. 2016 Apr 22;6:24597. doi: 10.1038/srep24597. Sci Rep. 2016. PMID: 27103053 Free PMC article.
-
Systematic exploration of Escherichia coli phage-host interactions with the BASEL phage collection.PLoS Biol. 2021 Nov 16;19(11):e3001424. doi: 10.1371/journal.pbio.3001424. eCollection 2021 Nov. PLoS Biol. 2021. PMID: 34784345 Free PMC article.
-
Membrane Penetration by Bacterial Viruses.J Virol. 2017 Jun 9;91(13):e00162-17. doi: 10.1128/JVI.00162-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28404851 Free PMC article. Review.
-
Bacteriophage-encoded functions engaged in initiation of homologous recombination events.Crit Rev Microbiol. 2009;35(3):197-220. doi: 10.1080/10408410902983129. Crit Rev Microbiol. 2009. PMID: 19563302 Review.
Cited by
-
DeepPBI-KG: a deep learning method for the prediction of phage-bacteria interactions based on key genes.Brief Bioinform. 2024 Sep 23;25(6):bbae484. doi: 10.1093/bib/bbae484. Brief Bioinform. 2024. PMID: 39344712 Free PMC article.
-
The protein interaction network of bacteriophage lambda with its host, Escherichia coli.J Virol. 2013 Dec;87(23):12745-55. doi: 10.1128/JVI.02495-13. Epub 2013 Sep 18. J Virol. 2013. PMID: 24049175 Free PMC article.
-
ATP serves as a nucleotide switch coupling the genome maturation and packaging motor complexes of a virus assembly machine.Nucleic Acids Res. 2020 May 21;48(9):5006-5015. doi: 10.1093/nar/gkaa205. Nucleic Acids Res. 2020. PMID: 32255177 Free PMC article.
-
Advancement in the development of single chain antibodies using phage display technology.PeerJ. 2024 Apr 10;12:e17143. doi: 10.7717/peerj.17143. eCollection 2024. PeerJ. 2024. PMID: 38618563 Free PMC article.
-
Protein-protein interactions of human viruses.Semin Cell Dev Biol. 2020 Mar;99:31-39. doi: 10.1016/j.semcdb.2018.07.018. Epub 2018 Jul 26. Semin Cell Dev Biol. 2020. PMID: 30031213 Free PMC article. Review.
References
-
- Abril AM, Marco S, Carrascosa JL, Salas M, Hermoso JM. Oligomeric structures of the phage phi29 histone-like protein p6. J Mol Biol. 1999;292:581–588. - PubMed
-
- Agirrezabala X, Martin-Benito J, Valle M, Gonzalez JM, Valencia A, Valpuesta JM, Carrascosa JL. Structure of the connector of bacteriophage T7 at 8A resolution: Structural homologies of a basic component of a DNA translocating machinery. J Mol Biol. 2005b;347:895–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

