Oxidized base damage and single-strand break repair in mammalian genomes: role of disordered regions and posttranslational modifications in early enzymes
- PMID: 22749145
- PMCID: PMC3531629
- DOI: 10.1016/B978-0-12-387665-2.00006-7
Oxidized base damage and single-strand break repair in mammalian genomes: role of disordered regions and posttranslational modifications in early enzymes
Abstract
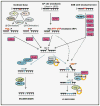
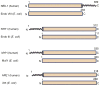
Oxidative genome damage induced by reactive oxygen species includes oxidized bases, abasic (AP) sites, and single-strand breaks, all of which are repaired via the evolutionarily conserved base excision repair/single-strand break repair (BER/SSBR) pathway. BER/SSBR in mammalian cells is complex, with preferred and backup sub-pathways, and is linked to genome replication and transcription. The early BER/SSBR enzymes, namely, DNA glycosylases (DGs) and the end-processing proteins such as abasic endonuclease 1 (APE1), form complexes with downstream repair (and other noncanonical) proteins via pairwise interactions. Furthermore, a unique feature of mammalian early BER/SSBR enzymes is the presence of a disordered terminal extension that is absent in their Escherichia coli prototypes. These nonconserved segments usually contain organelle-targeting signals, common interaction interfaces, and sites of posttranslational modifications that may be involved in regulating their repair function including lesion scanning. Finally, the linkage of BER/SSBR deficiency to cancer, aging, and human neurodegenerative diseases, and therapeutic targeting of BER/SSBR are discussed.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
References
-
- Dawson TL, Gores GJ, Nieminen AL, Herman B, Lemasters JJ. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am J Physiol. 1993;264:C961–7. - PubMed
-
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. - PubMed
-
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–15. - PubMed
-
- Mitra S, Hegde ML, Theriot CA, Das A, Hegde PM, Hazra TK. Complexity in repair of oxidative genome damage and its regulation. Proceedings of princess Takamatsu symposium; Tokyo, Japan. 2009.
-
- Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

